

| Received | : | Jul 15, 2022 |
| Accepted | : | Sep 01, 2022 |
| Published Online | : | Sep 02, 2022 |
| Journal | : | Neurology and Neurological Sciences: Open Access |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Nurullayev VH, Usubaliyev BT, Tagiyev DB. Coordination Compounds for Rheological and Physical-Chemical Regularity of Energy Consumption Decrease While Transporting Crude Oils. Nanosci Nanotechnol Open Access. 2022; 1(1): 1001.
Keywords: Complex compound; A layered porous structure; Thermal decomposition; benzenetetracarboxylic acid; A chemical formula.
For the first time, complex compound of zinc (II) 1,2,4,5-benzenetetracarboxylic acid with a porous structure was synthesized. Individuality and chemical formula of a complex compound was determined according to X-ray diffraction, elemental, IR spectroscopy and derivatographic analysis. The process of thermal decomposition of the resulting compound was also studied.
It is also found that, despite the fact that the parameters of the unit cell of the crystal are significantly different from the known complex, it retains its layered polymer and porous structure.
Recently high-viscosity oil fields, where non-Newtonian crudes are met, are rapidly developed. Non-Newtonian viscosity dependence on the flow rate imposes certain requirements to the transportation of such petroleum. To improve the rheological properties, the kinematic viscosity of the heaviest Azerbaijan oil from Muradhanlinskoye field, in particular, so that to facilitate pipeline transportation of the tank oil. Laboratory experiments and analysis of laboratory data proved the suitability of nanostructured coordination polymers for transporting high-viscosity oil from Azerbaijan Muradhanlinskoye field. This significantly reduces viscosity of heavy oil during transportation. Coordination polymer-based composites have been developed and tested. Use of composite solves a number of technological problems associated with the transport of high-viscosity oil.
Previously, we synthesized carboxylates of two main acids, in particular, phthalic and terephthalic acids. It has been established that they have a correspondingly zigzag and band like structure which give compounds of “host-guest” inclusion type [1 -5] with organic acids (acetic and formic).
It is also found out that formation of these types of connections is directly related to their structures, that is, when they are in contact with these acids due to their polymeric structures, acid molecules are positioned in interchain spaces. The number of included molecules depends on size and geometric forms of these molecules, that is, their clathrate formation is dependent on the size factor.
In addition to the size factor, pH medium is also of great importance. Depending on pH values, their structures change for easy clathrate formation [5]. We have also synthesized and decoded crystal structure of decahydrate complex of copper (II) with 1,2,4,5-benzenetetracarboxylic (pyromellitic) acid (Figure 1) [6].
Thus, the aim of this work is to study the complexation of pyromellitic acid, with further receipt of its non-bonded joints on its basis.
This work presents the results of the synthesis, physical and chemical and structural and chemical studies of complex compounds of zinc (II of) resulting in a weakly acidic medium (pH = 6.8).
Radiograph of the complex is shown in Figure 2.
As seen in Figure 2, the complex compound is highly crystalline and has a high symmetry. With X-ray indexing unit cell parameters were computed: a = 9,78, b = 19,7, c = 11,76Å.
From the crystal structure it is seen that the complex consists of polymeric nets of parallel planes (011). The acid anion for coordination impact uses all four carboxyl groups.
The composition of the crystalline compound coordinately bound with copper atoms of water molecules also includes two molecules of water of crystallization, which by means of hydrogen bonds covering all oxygen atoms bind the layers into a single unit in the form of 3D crystal structure. Wherein one layer of complexes (light) is slightly shifted in the plane (011) with respect to other layer systems (black). Based on the above mentioned, it can be assumed that in the absence of water molecules of crystallization, there would not occur displacement of layers. In this case, large pores in the carcass layers would lie on top of each other and large through columns with ability to include “guest” molecules would be formed. It should be noted that in this structure the interlayer space is also available for the inclusion of appropriate molecules.
Comparison of the parameters of the complex compound with parameters of the known complex compounds of copper (II) the crystal structure of which was decoded (a = 9,679 (5), b = 18,17 (2), c = 12,18 Å) showed that they differ respectively by 0.11; 1 and 0,42 Å. As it is seen, the parameters of a and b increase, whereas parameter of c decreases. These values are low, so at the first approach it would be found out that they are isostructural.
But the results of the elemental, IR spectroscopy and differential thermal analyzes have not confirmed these isostructural compounds, as elemental analysis results showed that the content of the complex is very different from the complex [1]. Elemental analysis results are presented in Table 1.
Table 1: The results of elemental analysis of the complex compounds of copper and zinc (II).
As it is seen from the table, the compositions of the compounds are very different from each other. The composition of the newly obtained compound has the Temperature, °C.
preliminary chemical formula Zn2(C6H2(COO)4)(H2O)4, whereas the chemical formula of the famous complex is Сu2(C6H2(COO)4)(H2O)10.
Termogravigramma of the complex compound of zinc (II) is shown in Figure 3. Decomposition of the complex compounds of zinc (II) begins at 90°C in the temperature range of 90-138°C and is accompanied by a shallow but clear endothermic effect at 110°C and corresponds to the removal of two molecules of water.
Experimental loss value of mass is 8 % (calculated 7.95%). Thereafter, in DTA curve there occurs the second endothermic effect in the temperature range of 138 - 180°C with maximum at 150°C, which corresponds to 1.5 moles of water removal. Experimental loss value of mass is 6% (calculated 5.96%).
Then there occurs the third fuzzy and shallow endothermic effect in the temperature range of 180 - 280°C with maximum at 240°C which corresponds to removal of an additional 0.5 moles of water. Here, experimental weight loss of the mass is 2% (calculated 1.99%). Anhydrous intermediate complex is stable up to 400°C which is extremely rare for complex compounds. At 400°C, first slowly, then with high speed decomposition of anhydrous complex takes place in the temperature range of 400 - 600°C with a single clear exothermic effect with the maximum 520°C. Here the experimental weight loss of the mass is 48% (calculated 48.15%). Since on the curve TG after complete decomposition is not observed weight increase, it can be concluded that oxidation of the zinc ion is due to oxygen atoms of the carboxyl groups. The final product is a ZnO. Experimental mass of the final product is 36% (calculated 35.95%). Below is a diagram of a solid phase transformation of complex compounds:
Since a weight increase is not observed on TG curve after complete decomposition, it can be concluded that oxidation of the zinc ion is due to oxygen atoms of the carboxyl groups. As the final product there remain ZnO. Mass of final experimental product is 36% (calculated 35.95%). Below is a diagram of a solid phase transformation of complex compounds:
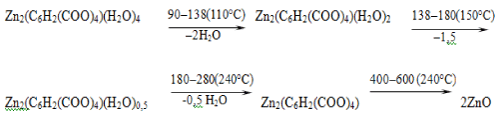
IR spectroscopic study also indicates that the frequency at 461; 534; 553 and 590 sm-1 refers to librational vibrations of water of crystallization or torsional vibrations of water molecules with limited interactions with neighboring atoms (Figure 4) [7].
Besides it, absorption bands are observed in IR spectrum of compound at 3550-3200 cm-1 (symmetric and asymmetric valent vibrations of OH) and at 1630 - 1600 cm-1 (deformation vibrations of HOH), which are characteristic for water of crystallization.
Besides it, absorption bands are observed in IR spectrum of compound at 3550-3200 cm-1 (symmetric and asymmetric valent vibrations of OH) and at 1630 - 1600 cm-1 (deformation vibrations of HOH), which are characteristic for water of crystallization.
Absorption bands at 1597, 1548, 1505 (νa) and 1457, 1401, 1337 cm1 (νs) refers to the carboxyl group of an acid anion [7]. Value of the difference value Δ (νa - νs) are respectively 140, 146 and 127 cm1 and it is significantly less than that of ionic compounds, but is in good agreement with the values of bidentate chelate complexes [8].
Thus, the central atom is coordinated to six. Coordination zinc (II) includes four oxygen atoms of two carbonyl groups and two oxygen atoms of water molecules. Coordination polyhedron is octahedron.
Figure 5 shows the structure of the estimated complex compound. As it is seen from the figure, the structure of the complex compound Zn2(C6H2(COO)4)(H2O)4 consists of alternating layers along the axis (011). The structure is porous and the size of pores is approximately 9 х 16 , as in [6]. The layers are stitched together due to hydrogen bonds formed by coordination of water molecules in different layers on the tops of octahedra (Figure 6). It is also possible to assume that the skeleton pores in the structure will be one above the other and, in this case, through columns for the available “guest molecules” will be generated. Thus, one can conclude that a series of non-bonded compounds having practical value can be synthesized on the basis of this compound.
The elemental composition of the obtained compound was defined by gas chromatography method by means of an analyzer CHN30E Carlo ERBA. The content of the metal was calculated on the basis of the weight loss curve by the quantity of oxide obtained after being heated on derivatograph up to 800°C. X-ray phase analysis was performed on the device Commander Sample ID (Coupled Two Theta/Theta) WL 1.54060.
IR spectra were recorded on a device SPECORD-MBO in 400-4000 cm1 area. Derivatograms recorded derivatograph on NETZSCH STA 449F3 STA449F3A-0836-M (range 21 / 10.0 (K / min) / 800).
The starting materials were C6H2(COO)4, Zn2(CH3COO)2 of qualification 4 (GOST 3759 - 75). The complex is prepared by reacting pyromellitic acid with zinc acetate at a stoichiometric ratio of 1: 2. The solution was refluxed until disappearance of the odor of acetic acid, filtered while hot and cooled to room temperature.
Upon cooling small transparent single crystals dropped from the solution which were filtered and washed for several times with warm distilled water, and were left to dry on filter paper at room temperature. Chemical composition of the complex compound was defined on the basis of the data obtained from phase X-ray, elemental, thermogravimetric and IR spectroscopic analysis.
It should be noted that these focal nanostructured polymers are a new generation of reagents that improve the rheological properties of heavy (nonyuton) crude oils [9,10,11].
Preparation of composition on the basis of polymer is as follows: at continuous hashing enter the measured quantity of a sulfanol after which dissolution, add polymer into the container with water, continuing to mix within 5 minutes. Process is carried out with an atmospheric pressure and room temperature.
The offered way is carried out as follows: enter the composition representing solution on the basis of nanostructural coordination polymer prepared by the known technique into oil (field of MuradkhanliAzerbaijan, Table 2).
The water composition of polymer mixes up with oil at a ratio solution to raw materials as 1÷ 5: 30. It is also necessary to pay attention that the place of input of reagent has to be located on a technological stream above that point in which it is necessary to provide reduction of viscosity.
Enter composition on the basis of polymer at a solution ratio to oil 1 ÷ 5: 30 into 300 ml (262,8 g) of oil. 0,1% are a part of composition on the basis of water solution of polymer (masses.) sulfanola, 0,5% (masses.) polymer, the rest-technical water. Comparative data on influence of a ratio of composition to oil on indicators of its kinematic viscosity are provided in table 3. Apparently from the obtained data, the best results have been received at a composition ratio to oil as 4: 30. Proceeding from it, this ratio is accepted as optimum for further examples. At the same time the kinematic viscosity of oil decreases to value 44,86 of mm2/sec.
Table 3: Physical and chemical characteristics of oil of the field of Muradkhanli before and after reagent addition.
Apparently from the presented examples, the offered way favourably differs in efficiency to use the polymer of containing of compositions which receiving is based on application of the components made commercially, the polymers which don't have optical and geometrical isomers, and for achievement of considerable improvement of rheological properties of hydrocarbonic system (decrease in kinematic viscosity) there is enough use of insignificant amount of nanostructural coordination polymer that allows to improve not only conditions, but also profitability of transportation high-viscosity oil.
The authors gratefully acknowledge the research council of State Oil Company of the Azerbaijan Republic (SOCAR) and Azerbaijan State University of Oil and Industry.
This work carried out with the financial support of the Foundation for the Development of Science under the President of the Republic of Azerbaijan - Grant No. EIF / GAM-3-2014-6 (21) -24/09/04.
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.