Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Short Commentary
- |
- Open Access
- |
- ISSN: 2639-4383
Which is the Best Risk Stratification Approach for Patients with Ventricular Arrhythmias in the Absence of Apparent Structural Heart Disease?
- Konstantinos A Gatzoulis;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Constantina Aggeli*;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Yannis Dimitroglou;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Stefanos Archontakis;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Petros Arsenos;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Ioannis Vlaseros;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Polichronis Dilaveris;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Christos-Konstantinos Antoniou;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Dimitris Patsourakos;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Skevos Sideris;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Konstantinos Tsioufis;
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece
- Dimitris Tousoulis
- Department of Cardiology, National and Kapodistrian University of Athens, Hippokration General Hospital, Athens, Greece

| Received | : | Aug 01, 2020 |
| Accepted | : | Sep 07, 2020 |
| Published Online | : | Sep 10, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Gatzoulis KA, Aggeli C, Dimitroglou Y, Archontakis S, Arsenos P, et al. Which is the Best Risk Stratification Approach for Patients with Ventricular Arrhythmias in the Absence of Apparent Structural Heart Disease?. Ann CardiolVasc Med. 2020: 3(1); 1026.
Short commentary
There is tremendous interest in identifying those at risk among patients presenting with premature ventricular complexes in the absence of apparent Structural Heart Disease (SHD), a frequently met and highly debatable group of individuals [1]. Long-term prognosis of patients presenting with significant burden of isolated and/or complex Ventricular Arrhythmias (VA) in the absence of detectable SHD on the formal transthoracic echocardiography was considered for years relatively benign and comparable to that of the general population when Coronary Artery Disease (CAD) had been excluded [2]. However, according to early findings by Abdalla et al, patients with VA detected on a rest 2-minute electrocardiogram have increased risk for Sudden Cardiac Death (SCD) not attributable to CAD [3]. In this research work as well as in later studies, a baseline echocardiogram had not been performed [4,5]. Moreover, most studies included in the meta-analysis of Atakite et al, did not also include many other clinical confounders and SCD risk factors [6]. Even in some later studies by Lin et al, supporting the view that multiform VA or Nonsustained Ventricular Tachycardia (NSVT) in patients with normal hearts were related to worse cardiovascular outcome, a comprehensive echocardiographic evaluation had not been performed [7,8].
As has been shown by a prospective study performed in our institution in patients with idiopathic right ventricular ectopy in the absence of known SHD, the long term prognosis was excellent provided conditions such as unrecognized Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), tachycardiomyopathy or production/conduction abnormalities were appropriately and timely taken care of [9]. The majority of these patients underwent also signal averaged electrocardiography (SAECG) with some of them having invasive Electrophysiology (EPS) and Cardiac Magnetic Resonance (CMR) as well, with a variety of abnormal imaging and electrophysiological parameters being detected. Similarly, Adreini et al performed CMR in patients with normal echocardiographic analysis and found a significant proportion (25%) of the 946 patients studied to have some evidence of underlying SHD, most commonly myocarditis. The important question whether these patients with the unrecognized SHD are at risk for major arrhythmic events in the long run, has not been answered in this prospective single center study [10]. Hence, it remains to be answered, whether increased risk of cardiac death including SCD in patients with VA can be attributed to an unrecognized SHD or to the sole presence of these arrhythmias that not infrequently subside over time [9].
Evaluation should begin with a thorough history regarding pre-syncope or syncope and family history for sudden cardiac death or cardiomyopathy [11]. Concerning findings in the 12-lead ECG, signs of ischemic heart disease (regional Q waves, or poor progress of R wave), hypertrophic cardiomyopathy (left ventricular hypertrophy, deep narrow Q waves in lateral leads, T-wave abnormalities, QRS axis deviation), non-ischemic cardiomyopathy (QRS fragmentation, S/R ratio in V6 lead ≥ 0.25), ARVC (epsilon wave with T wave inversion in the right precordial leads), prolonged repolarization or Brugada ECG pattern should be sought [9,12].
A signal-averaged ECG (SAECG) should be performed in all VA patients. SAECG have been shown to significantly predict arrhythmic SCD in ischemic heart disease and dilated cardiomyopathy [13]. In patients without detectable SHD, positive SAECG can become a stimulus for the clinician to look for subclinical ischemic heart disease, DCM or ARVC.
Although conventional transthoracic echocardiography offers adequate data concerning the structural and functional properties of the heart, newer advanced modalities have an additional diagnostic role. Several papers support the use of contrast agents in order to enhance the diagnostic approach of transthoracic echocardiography [14]. Indeed, apart from wall motion abnormalities, and a better assessment of Left Ventricular (LV) dimensions and Left Ventricular Ejection Fraction (LVEF), contrast echocardiography may also detect trabeculations and apical hypertrophic cardiomyopathy in patients without evidence of SHD at baseline echocardiography [14-16]. Moreover, speckle tracking echocardiography as a novel modality, has been shown to be superior to standard 2D-echocardiography for detecting subclinical LV dysfunction at early stages [17]. Global Longitudinal Strain (GLS) is worse in relatives of DCM patients without SHD and may predict future LVEF deterioration [18]. Reduced absolute GLS has been related to worse prognosis in patients with SHD even when LVEF is within normal limits [19]. Among VA patients, a comprehensive analysis of Right Ventricular (RV) dimensions and systolic function with calculation of right ventricular strain and mechanical dispersion may also detect patients with ARVC at early stages [9,20-22]. Contrast echo can also provide additional information for the RV, with visualization of small aneurysms or detection of wall motion abnormalities. Such advanced echocardiographic techniques have also been proposed for the risk stratification approach for mitral valve prolapse patients [23,24].
VA induced cardiomyopathy may be seen in patients with high VA burden, with PVC QRS duration >150ms and VA of epicardial origin [1]. In such patients further investigation for the presence of subclinical SHD with the previously mentioned techniques is justified, especially when lifestyle modifications and medical treatment with beta-blockers or calcium channel blockers is ineffective and ablation therapy has failed [1,25].
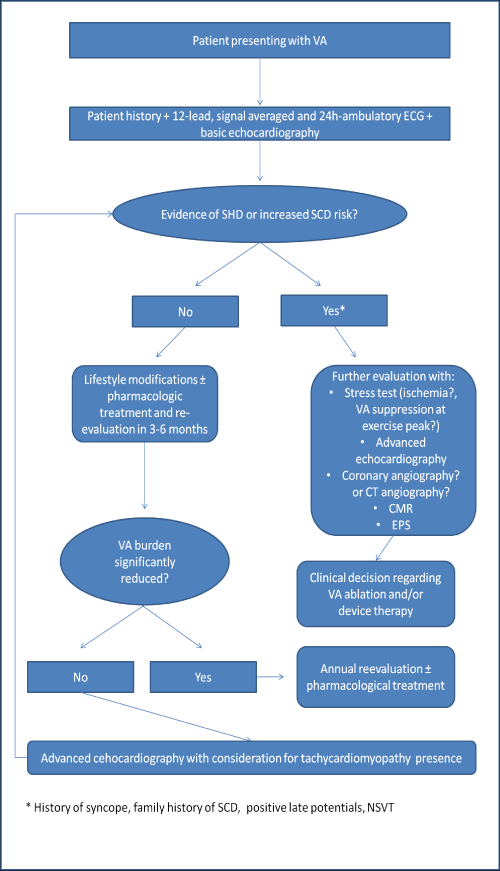
Figure: Proposed risk stratification and management approach.
VA: Ventricular Arrhythmias; ECG: Electrocardiography; SHD: Structural Heart Disease; SCD: Sudden Cardiac Death; CT:Computed Tomography; CMR: Cardiac Magnetic Resonance; EPS: Electrophysiology Study; NSVT:Nonsustained Ventricular Tachycardia.
To answer the question who are the patients in need for CMR investigation, a not widely available and difficult to interpret imaging technique, one should take into account the easily derived evidence from history (i.e., pre and syncope, family history of sudden cardiac death or hereditary cardiomyopathy) and the 12 lead, signal averaged and ambulatory electrocardiography (T wave inversion, late potentials, nonsustained or sustained VT). To go one step forward the question of whether such patients should be further risk stratified with EPS when some SHD has been recognized on CMR or the advanced echocardiographic techniques, is a matter of additional good clinical practice and future research. A proposed algorithm for the risk stratification and management of the cardiac patient presenting with VA in the absence of obvious SHD on the initial screening, is summarizing the above in the text Figure.
References
- Marcus GM. Evaluation and Management of Premature Ventricular Complexes. Circulation. 2020; 141: 1404-1418.
- Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, et al. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985; 312: 193-197.
- Abdalla ISH, Prineas RJ, Neaton JD, Jacobs DR, Crow RS. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol. 1987; 60: 1036-1042.
- Engel G, Cho S, Ghayoumi A, Yamazaki T, Chun S, et al. Prognostic significance of PVCs and resting heart rate. Ann Noninvasive Electrocardiol. 2007;12: 121-129.
- Cheriyath P, He F, Peters I, Li X, Alagona P, et al. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the atherosclerosis risk in communities [ARIC] study). Am J Cardiol. 2011;107:151-155.
- Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am. J. Cardiol. 2013;112:1263-1270.
- Lin CY, Chang SL, Lin YJ, Lo LW, Chung FP, et al. Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol. 2015;180: 80-85.
- Lin CY, Chang SL, Chung FP, Chen YY, Lin YJ, et al. Long-term outcome of non-sustained ventricular tachycardia in structurally normal hearts. PLoS One. 2016; 11
- Gatzoulis KA, Archontakis S, Vlasseros I, Tsiachris D, Vouliotis A, et al. Complex right ventricular outflow tract ectopy in the absence of organic heart disease. Results οf a long-term prospective observational study. Int J Cardiol. 2014; 172: e351-3.
- Andreini D, Dello Russo A, Pontone G, Mushtaq S, Conte E, et al. CMR for Identifying the Substrate of Ventricular Arrhythmia in Patients With Normal Echocardiography. JACC Cardiovasc Imaging. 2020; 13: 410-421.
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary. Circulation. 2018;138: e210-e271.
- Gorenek B, Fisher JD, Kudaiberdieva G, Baranchuk A, Burri H, et al. Premature ventricular complexes: diagnostic and therapeutic considerations in clinical practice: A state-of-the-art review by the American College of Cardiology Electrophysiology Council. J. Interv. Card. Electrophysiol. 2020; 57: 5-26.
- Gatzoulis KA, Arsenos P, Trachanas K, Dilaveris P, Antoniou C, et al. Signal-averaged electrocardiography: Past, present, and future. J. Arrhythmia. 2018; 34: 222-229.
- Aggeli C, Felekos I, Tsiamis E, Toutouzas K, Stefanadis C. Contrast Echocardiography: An Update on Clinical Applications. Curr Pharm Des. 2012; 18: 2200-2206.
- Gaibazzi N, Tuttolomondo D, Rabia G, Lorenzoni V, Benatti G, et al. Standard echocardiography versus very-low mechanical index contrast-imaging: left ventricle volumes and ejection fraction multi-reader variability and reference values in a subgroup with no risk factors or cardiac disease. Heart Vessels. 2020; 35: 544-554.
- Larsson MK, Da Silva C, Gunyeli E, Ilami AA Bin, Szummer K, et al. The potential clinical value of contrast-enhanced echocardiography beyond current recommendations. Cardiovasc Ultrasound. 2016; 14: 2.
- Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging. 2018; 11: 260-274.
- Verdonschot JAJ, Merken JJ, Brunner-La Rocca HP, Hazebroek MR, Eurlings CGMJ, et al. Value of Speckle Tracking–Based Deformation Analysis in Screening Relatives of Patients With Asymptomatic Dilated Cardiomyopathy. JACC Cardiovasc Imaging. 2020; 13: 549-558.
- Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014; 100: 1673-1680.
- Cappelletto C, Stolfo D, De Luca A, Pinamonti B, Barbati G, et al. Lifelong arrhythmic risk stratification in arrhythmogenic right ventricular cardiomyopathy: Distribution of events and impact of periodical reassessment. Europace. 2018;20:f20–f29.
- Leren IS, Saberniak J, Haland TF, Edvardsen T, Haugaa KH. Combination of ECG and Echocardiography for Identification of Arrhythmic Events in Early ARVC. JACC Cardiovasc Imaging. 2017; 10: 503-513.
- Pieles GE, Grosse-Wortmann L, Hader M, Fatah M, Chungsomprasong P, et al. Association of echocardiographic parameters of right ventricular remodeling and myocardial performance with modified task force criteria in adolescents with arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging. 2019;12: e007693.
- Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart. 2019; 105: 144-151.
- Ermakov S, Gulhar R, Lim L, Bibby D, Fang Q, et al. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart. 2019; 105: 1063-1069.
- Latchamsetty R, Bogun F. Premature Ventricular Complex–Induced Cardiomyopathy. JACC Clin. Electrophysiol. 2019; 5: 537-550.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.