Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Use of Pulmonary Vasodilator Therapy to Manage Pulmonary Arterial Hypertension with Associated Large Pulmonary Artery Aneurysms and Pulmonary-Systemic Artery Collaterals in the setting of Gerbode Defect
- Humna Abid Memon;
- Pulmonary Critical Care Fellow, Department of Pulmonary Critical Care Medicine, University of Cincinnati, Cincinnati, OH, USA
- Derek Gibbs;
- Internal Medicine Resident, Department of Internal Medicine, University of Cincinnati, Cincinnati, OH, USA
- Jean M Elwing*
- Professor of Medicine, Department of Pulmonary Critical Care Medicine, University of Cincinnati, Cincinnati, OH, USA

| Received | : | Sep 22, 2020 |
| Accepted | : | Oct 23, 2020 |
| Published Online | : | Oct 23, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Abid Memon H, Gibbs D, Elwing JM. Use of Pulmonary Vasodilator Therapy to Manage Pulmonary Arterial Hypertension with Associated Large Pulmonary Artery Aneurysms and Pulmonary-Systemic Artery Collaterals in the setting of Gerbode Defect. Ann Cardiol Vasc Med. 2020: 3(1); 1031.
Keywords: Gerbode defect; Pulmonary arterial hypertension; Pulmonary-systemic collateral.
Abstract
Gerbode defect, described as an abnormal connection between the left ventricle and right atrium, is an extremely rare form of intra-cardiac shunt. Diagnosis of Gerbode is difficult as the shunt is often misdiagnosed initially as Pulmonary Arterial Hypertension (PAH) or tricuspid regurgitation. While diagnosis of Gerbode can be onerous, management of these patients is even more challenging, as their case can be complicated by the development of PAH.
Treatment of patients with Gerbode is usually sought by surgically or percutaneously correcting the defect. Amongst those who are unable to undergo repair, development of PAH as a result of underlying congenital heart disease is a possibility. Historically, PAH in these patients has been managed with pulmonary vasodilators. However, use of these medications to treat PAH in patients with Gerbode in the presence of complicating large pulmonary artery aneurysms and pulmonary-systemic artery collaterals has not been described before.
This report discusses the case of a 28-year-old female diagnosed initially with idiopathic PAH. On subsequent re-evaluation, she was found to have congenital Gerbode defect with resultant PAH along with pulmonary artery aneurysm and extra-cardiac shunting due to pulmonary-systemic artery collaterals. Through this case we highlight the diagnostic difficulties that are commonly encountered in patients with Gerbode defect. Furthermore, we seek to describe the safe utilization of pulmonary vasodilators for the management of PAH secondary to Gerbode in the challenging setting of concurrent large pulmonary artery aneurysms and pulmonary-systemic collaterals.
Introduction
With improved diagnostic modalities, monitoring, and interventions the detection of structural heart defects is steadily increasing in the adult population. Left Ventricle (LV) to right atrium (RA) shunts, termed as Gerbode defect, are quite rare as they constitute less than 1% of all congenital heart defects and less than 0.8% of intra-cardiac shunts [1]. Increase in the incidence of endocarditis and surgical cardiac interventions, such as valve replacement (complications of which lead to LV-RA shunts), have led to acquired form of Gerbode defect becoming more common than its congenital counterpart [1,2].
The low incidence of Gerbode defect compounded by difficulty in diagnosing the shunt on the most utilized modality of imaging (Echocardiogram (ECHO)) often leads to delayed and ineffective care of these patients. When identified correctly and timely, the condition is usually treated with surgical or percutaneous correction [1,3-5]. Due to a sustained high pressure state within the pulmonary circulation, those who are unable to undergo repair of the defect can subsequently develop pulmonary arterial hypertension (PAH) [6]. While there are accounts of PAH in patients with congenital heart defects being managed with pulmonary vasodilators, use of these medications in presence of concurrent large pulmonary artery aneurysms and pulmonary-systemic artery collaterals has not been reported [7].
Herein, we describe the case of a young female diagnosed initially as idiopathic PAH. On re-evaluation she was later found to have PAH secondary to congenital Gerbode defect along with large pulmonary artery aneurysms and extra-cardiac shunting due to pulmonary-systemic artery collaterals. Unlike prior reported cases, her symptoms were managed successfully with a combination of pulmonary vasodilators.
Case presentation
A 28-year-old female with a past medical history of PAH presented to the pulmonary clinic for evaluation of persistent dyspnea on exertion that she had been experiencing since childhood. Investigation for this complaint in the past had been conducted with an extensive workup, including pulmonary function testing that had demonstrated normal lung volumes with decreased diffusion capacity. An ECHO had also been performed and had revealed normal LV function with moderately enlarged Right Ventricle (RV) and Pulmonary Artery (PA) Systolic Pressure (PASP) of 68 mmHg with a right to left shunt, thought to be consistent with Patent Foramen Ovale (PFO). Given ECHO findings, Right Heart Catheterization (RHC) at the age of 18 years was performed with findings notable for a Right Atrial Pressure (RAP) of 6 mmHg, right ventricular pressure (RVP) of 100/11 mmHg, Mean Pulmonary Pressure (mPAP) of 70 mmHg, pulmonary wedge pressure (PCWP) of 10 mmHg, Cardiac Output (CO) of 3.8 L/min, Cardiac Index (CI) of 2.3 L/min/m2, and Pulmonary Vascular Resistance (PVR) of 15.8 woods. Her hemodynamics as well as her medical history along with aforementioned detailed evaluation were consistent with idiopathic PAH at that time and therapy was hence initiated with sildenafil 20mg thrice daily and bosentan 125 mg twice daily.
The patient’s dyspnea improved gradually with PAH therapies. However, she subsequently developed systemic hypertension and supraventricular tachycardia. Bosentan was thus discontinued due to concern for intolerance. Repeat RHC was performed one year after her previous one and revealed following hemodynamics: RAP 4 mmHg, RVP 82/9 mmHg, mPAP 56 mmHg, PCWP 15 mmHg, and PVR 15.8 woods. Given persistent elevation in mPAP, sildenafil was increased to 40 mg thrice daily and ambrisentan 10 mg daily was initiated.
While being maintained on the above regimen, close monitoring was conducted with frequent clinic visits, along with six-minute walk (6MWT) tests, all of which demonstrated stability. Serial ECHOs were also obtained during this time and were remarkable for stable RV size and function with persistent right to left shunt.
After several years of stable symptoms and positive response to dual oral therapy with sildenafil and ambrisentan, the patient began to decline and developed worsening shortness of breath (SOB). Repeat ECHO at that time demonstrated worsening dilation and reduced systolic function of the RV. Significant reduction in 6 MWT was concurrently noted. Due to these developments, repeat RHC was obtained and was notable for an increase in mPAP to 71 mmHg along with PCWP of 10 mmHg and PVR of 11.2 Woods. In light of worsening hemodynamics, the patient was started on therapy with Intravenous (IV) epoprostenol. This was discontinued shortly thereafter as she developed significant medication side effects. Following this, the patient was transitioned to Subcutaneous (SC) treprostinil which was then slowly increased to a dose of 30 ng/kg/min.
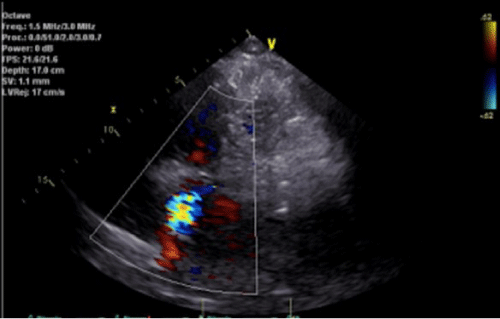
Despite up titration of treprostinil, patient’s dyspnea and 6 MWT continued to worsen. Re-evaluation with an ECHO (Figure 1) demonstrated a moderately dilated RV with decreased systolic function along with a predominant systolic jet on color Doppler that was directed perpendicularly to the RV inflow tract. This jet was observed to be originating in the superior portion of the interventricular septum and terminating in the RA, raising concerns for left to right intra-cardiac shunt. Transesophageal ECHO was obtained and confirmed a ventricular septal defect in the atrioventricular septum consistent with Gerbode Defect and resultant shunt with left to right gradient of at least 50 mmHg.
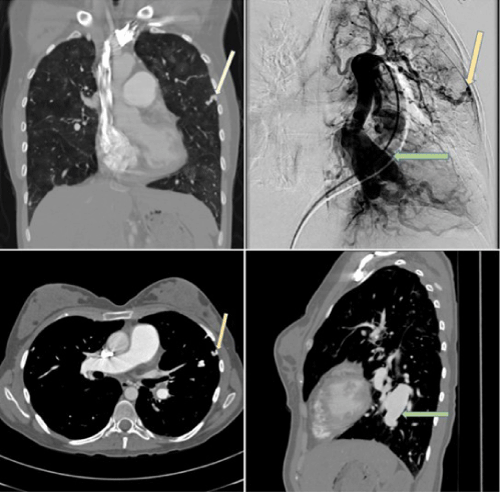
Shortly thereafter she began experiencing episodic hemoptysis. Computed tomography of chest with contrast was thus done (Figure 2) and revealed prominent lower lobe PA aneurysms. Furthermore, branches of the pulmonary arteries extending to periphery of lung and establishing collaterals with the systemic circulation were also noted. Pulmonary angiogram was performed and confirmed presence of the same aneurysms and pulmonary-systemic artery collaterals (Figure 2). These findings were deemed to be the etiology for the patient’s hemoptysis and most likely the result of long-standing PAH.
In light of the new finding of intra-cardiac shunt and worsening symptoms on sildenafil, ambrisentan, and SC treprostinil, repeat RHC was performed and revealed following hemodynamic parameters: mPAP 82 mmHg, left ventricular end diastolic pressure (LVEDP) 6 mmHg and PVR 10.8 woods. Due to worsening mPAP, functional status, and patient’s inability to tolerate IV epoprostenol, sildenafil, and ambrisentan were continued and SC treprostinil was slowly titrated up to 40 ng/kg/min. simultaneously, expedited transplant evaluation was initiated.
While being assessed for transplant, patient developed severe infusion site pain, rendering her unable to tolerate SC medication. SC treprostinil was thus discontinued and transitioned to oral treprostinil. The dose of sildenafil was increased as well. After being treated with this regimen for several months without improvement in symptoms, oral treprostinil was transitioned to IV treprostinil. While maintaining the patient on sildenafil and ambrisentan, the dose of treprostinil was then gradually increased as tolerated. The patient remained on triple therapy with IV treprostinil, sildenafil and ambrisentan with NYHA class III symptoms. She was subsequently listed and underwent successful double lung transplant with congenital heart disease repair.
Figure 1: Parasternal short axis view demonstrating shunt (Gerbode Defect) between Right Atrium (RA) and Left Ventricle (LV).
Figure 2: Computed Tomography (CT) pulmonary angiography demonstrating multifocal areas of PA dilatation (green arrow) and tortuous enhancing branches of PA extending to pleura (yellow arrow), raising concerns for PA aneurysms and PA-systemic artery fistulas, respectively. Pulmonary angiogram obtained for further evaluation (upper right picture) was consistent with the same findings.
Discussion
Congenital LV-RA shunt was first described in 1838 by Thurman during an autopsy [8]. In 1958 Gerbode described the closure of such a defect in five patients and had his name ascribed to the condition [9]. Since then, despite more reported cases, Gerbode still remains one of the rarest causes of congenital septal defects.
The case presented here aims to highlight the challenges faced in appropriate diagnosis and management of a woman with chronic dyspnea due to Gerbode defect and its associated complications. At the age of 18 years, she was mistakenly diagnosed with severe PAH. It was not until eight years later, that she underwent an ECHO and was found to have a septal defect with LV-RA shunt. This finding in the setting of no prior history of endocarditis, cardiac surgery, or other typical risk factors, was consistent with a congenital Gerbode defect and had likely been the reason for her chronic SOB.
Patients with Gerbode defect can present with a variety of symptoms that are dependent on the severity and size of the shunt. These can range from being asymptomatic to developing dyspnea, signs and symptoms of heart failure and sometimes even death [1,10]. Given the plethora of presentations that can mimic other common diseases, along with the fact that ECHO, which is usually the first step in evaluation, has a sensitivity of only 66% in detecting this defect, diagnosis of Gerbode can be often difficult [2,11]. As demonstrated by review of literature and our case, it is not uncommon for patients with Gerbode defect to be misdiagnosed as tricuspid regurgitation (TR) and/or PH [12-14]. The primary reason for this is that the defect in these patients originates in close proximity to the annulus of the tricuspid valve, thereby leading to a shunt which creates a turbulent jet with a high-pressure gradient that is often wrongfully identified as TR [9,15]. Given the limitations of ECHO in detecting the shunt, as seen in our case, transesophageal echocardiography often has to be employed to confirm its presence, as it carries a higher sensitivity than ECHO [1,16].
In our case, it is important to know that the patient underwent multiple prior ECHOs which were unable to identify this defect over an eight-year period. Of note, during the same time, she was seen by five different hospital systems, including a large pediatric center as well as pulmonary transplant center. This demonstrates not only the complexity of this case but the difficulty that even experienced physicians can encounter, due to the aforementioned reasons, in diagnosing this condition. Furthermore, this case also underscores the importance of including Gerbode defect within the differential for a young patient with ECHO findings that may be consistent with severe PAH and/or TR.
Historically, Gerbode defect has been usually treated with surgical or percutaneous correction [1,3-5]. If left untreated, as demonstrated by our patient, it can eventually lead to development of severe PAH. It should be noted that the finding of cardiac defect and shunt on ECHO, eight years after her first RHC, was initially thought to be an incidental finding, as the shunt had a relatively low gradient of 50 mmHg. However, it was later acknowledged that Gerbode defect likely played an important role in the development of PAH, with the shunt and its gradient now being blunted by the rising right sided pressure which had likely led the pressure within the pulmonary vessels to become almost equivalent to that of her systemic circulation. For the same reason in the event of no further escalation of treatment, there is concern that this patient will progress to worsening right heart failure.
Given the degree of PAH, development of vascular pruning, aortopulmonary shunting along with multiple pulmonary aneurysms, definitive therapy for our patient would be a bilateral pulmonary transplant. As she presented with severe PAH and resultant life limiting symptoms, she was treated with pulmonary vasodilators and had an initial robust response, both hemodynamically and symptomatically.
The use of pulmonary vasodilators to manage PAH associated with congenital heart defects like Gerbode, while not unique by itself, was however made extremely challenging in our patient by the presence of associated pulmonary-systemic artery collaterals [7]. This is because the use of vasodilators in such patients is accompanied by a theoretical risk of worsening the shunt across these collaterals. It is for this reason that extreme caution and close monitoring were undertaken while vasodilators were up titrated. However, as noted from our case, pulmonary vasodilators can be used safely in these patients, if employed with precaution.
In this patient with left to right shunt, the Gerbode defect and collaterals between the pulmonary systemic circulation had created a complex hemodynamic state which warranted maintenance of the delicate balance of right and left sided pressures so as to avoid exacerbation of left to right shunting. Over time, as anticipated, our patient’s disease advanced with worsening PAH, hemodynamics, and symptoms. Furthermore, her treatment, as noted above, was limited by medication intolerance and side effects. In light of this, after significant consideration by the patient and her family, she was listed and underwent definitive treatment for her underlying condition through successful double lung transplant and repair of underlying congenital heart defect.
References
- Saker E, Bahri G N, Montabano M J, Johal J, Graham R, et al. Gerbode defect: A comprehensive review of its history, anatomy, embryology, pathophysiology, diagnosis and treatment. J Saudi Heart Assoc. 2017; 29: 283-292.
- Yuan S. Expert review Left ventricular to right atrial shunt (Gerbode defect): Congenital versus acquired. Pwki. 2014; 3: 185-194.
- Kelle AM, Young L, Kaushal S, Duffy E, Anderson R, et al. The Gerbode defect: The significance of a left ventricular to right atrial shunt. Cardiol Young. 2009; 19: 96-99.
- Prifti E, Ademaj F, Baboci A, Demiraj A. Acquired Gerbode defect following endocarditis of the tricuspid valve: a case report and literature review. J Cardiothorac Surg. 2015; 10: 115.
- Yacoub MH, Mansur A, Towers M, Westbury H. Bacterial endocarditis complicating left ventricle to right atrium communication. Br J Dis Chest. 1972; 66: 78-82.
- Tukaye D, Craft J, Auseon A, Patel D. An elusive quest to explain the worsening pulmonary hypertension in a case of known stable restrictive ventricular septal defect. J Am Coll Cardiol. 2015; 65: A706.
- Countouris M, Jeyabalan A, Caldwell J, Lee J, Hickey G. Primary Presentation of Pulmonary Hypertension in the Peripartum. J Am Coll Cardiol Case Rep. 2020; 2: 125-130.
- Thurnam J. On aneurisms of the heart with cases. Med Chir Trans. 1838; 21: 187.
- Gerbode F, Hultgren H, Melrose D, Osborn J. Syndrome of left ventricular-right atrial shunt; successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg. 1958; 148: 433-446.
- Sinisalo JP, Sreeram N, Jokinen E, Qureshi S. Acquired left ventricular-right atrium shunts. Eur J Cardiothorac Surg. 2014; 39: 500–506.
- Dzwonczyk T, Davidson WR. Jr. The spectrum of left ventricular-right atrial communications in the adult: Essentials of echocardiographic assessment. J Am Soc Echocardiogr. 1995; 8: 263-269.
- Rehrani F, Movahed M. How to prevent echocardiographic misinterpretation of Gerbode type defect as pulmonary arterial hypertension. European Journal of Echocardiography, 2007; 8: 494-497.
- Xhagija N, Prifti E, Allajbeu I, Sula F. Gerbode defect following endocarditis and misininterpreted as severe pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010; 8: 44.
- Garg R, Garcia R, Cubeddu R. Gerbode defect misinterpreted at pulmonary hypertension. J Cardiol Cases. 2012; 7: 34-36.
- Yuan Shi-Min. Left ventricular to right atrial shunt (Gerbode defect): Congenital versus acquired. Postepy Kardiol Interwencyjnej. 2014; 10: 185-194.
- Bustamante S, Cheruku S. Intraoperative transesophageal echocardiography to evaluate a Gerbode defect. Colombian Journal of Anesthesiology. 2017; 45: 147-150.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.