Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case Report
- |
- Open Access
- |
- ISSN: 2639-4383
Unusual “Bail-Out” Method for Management of Distal Coronary Perforation
- Plamen Krasimirov Krastev;
- UMHAT “St. Ekaterina”-Sofia, Medical University of Sofia, Bulgaria.
- Stefan Naydenov Naydenov*;
- Department of Internal Diseases “Prof. St. Kirkovich”, Medical University of Sofia, Bulgaria.
- Nikolay Margaritov Runev;
- Department of Internal Diseases “Prof. St. Kirkovich”, Medical University of Sofia, Bulgaria.
- Emil Ivanov Manov
- Department of Internal Diseases “Prof. St. Kirkovich”, Medical University of Sofia, Bulgaria.

| Received | : | Oct 29, 2020 |
| Accepted | : | Nov 25, 2020 |
| Published Online | : | Dec 02, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Krastev PK, Stefan NN, Runev NM, Manov EI. Unusual “Bail-Out” Method for Management of Distal Coronary Perforation. Ann Cardiol Vasc Med. 2020: 3(1); 1034.
Keywords: Iatrogenic; Non-surgical; Rupture; Tamponade; Treatment.
Abstract
Coronary artery perforations occur in ~0.2-0.5% of all percutaneous coronary interventions (PCI): up to 60% of these patients need surgical treatment.
We present an unusual “bail-out” method for non-surgical management of acute distal coronary perforation. It was applied first in a 73-old patient, underwent an elective coronary intervention for high-grade stenosis in a tertiary hospital. The PCI was complicated by a distal coronary perforation, followed by cardiac tamponade and cardiac arrest.
We managed this situation successfully, applying a “plugging” matrix, crafted on site by our team from gelatin sponge, contrast and saline. After 2 transcatheter applications of this matrix at the perforation site, bleeding completely stopped, thus avoiding emergent surgical treatment. The patient fully recovered and was discharged 5 days later. The follow-up coronary angiography 3 months later confirmed the good result of our treatment and its relevance in critical situations of distal coronary perforations.
Introduction
Coronary Artery Perforation (CAP) is a life-threatening condition, occurring in ~0.2-0.5% of all patients undergoing percutaneous coronary interventions (PCI) [1-4]. This percentage is much higher when more aggressive techniques are used: directional coronary atherectomy, laser coronary angioplasty, rotablation, etc [5-8]. Despite the introduction of new interventional technologies, such as coated stents and embolization coils, surgery remains standard of care for many patients with CAP (up to 60%) because most of the non-surgical methods are inefficient in distal CAPs [2,3,8].
This gave us grounds to a present a case of a distal CAP, complicated by cardiac tamponade, in which we successfully used a unusual, effective and safe non-surgical “bail-out” method to manage this emergent condition. We present the following case in accordance with the CARE reporting checklist. A written informed consent has been obtained from the subject for publication of his data.
Case presentation
A 73-old male patient with chronic coronary syndrome, clinically manifested as stable angina III class according the Canadian classification, not managed by optimal drug treatment, was hospitalized for elective invasive coronary angiography. Previous coronary imaging in 2012 showed 2-vessel Coronary Artery Disease (CAD), with stenosis of the left anterior descending artery (LAD), the circumflex artery (RCx) and the first obtuse marginal branch (OM1): RCx and OM1 were revascularized with Drug-Eluting Stents (DES). This patient had the following concomitant diseases: arterial hypertension, hypercholesterolemia and type 2 diabetes, all of them diagnosed ~15 years ago and not well-controlled for most of the time.
The physical examination at the current admission showed regular heart rate ~60 beats/min, normal heart sounds without added murmurs, mean Blood Pressure (BP) from 3 measurements 132/74 mmHg, normal, well-filled peripheral arterial pulse.
The electrocardiography showed sinus rhythm ~60 beats per minute, left axis deviation and AV-block I degree. The Echocardiography (Echo) revealed normal sizes and volumes of the cardiac chambers, preserved left-ventricular segmental kinetics with ejection fraction 62% (by Simpson’s rule), mild degenerative changes of the aortic and mitral valves without valvular dysfunction.
Coronary angiography
The coronary imaging was performed through right radial artery access using 6F sheath. It showed 3-vessel CAD: 1. High-graded stenosis (~85%) in the middle segment of LAD with significant calcification of the plaque; 2. Complete chronic stent-thrombosis of RCx with co-lateral blood supply (Rentrop III grade) from the right coronary artery (RCA). The stent of OM1 was patent, with minimal in-stent proliferation; 3. Proximal RCA stenosis (~80%).
Coronary revascularization and complications
We cannulated the left main coronary artery with XB 3.5/6F catheter, overcame the high-graded LAD stenosis with 0.014” Runthrough® NS PTCA guide wire (Abbott) and reached LAD periphery. The next step was pre-dilation with a balloon Ryujin™ Plus 2.5/20 mm (Laeka JV Co Ltd.) applying 12-14 atmospheres pressure, followed by DES implantation (Orsiro 3.0/35 mm, Biotronik Ltd.), remodeled to 3.2 mm.
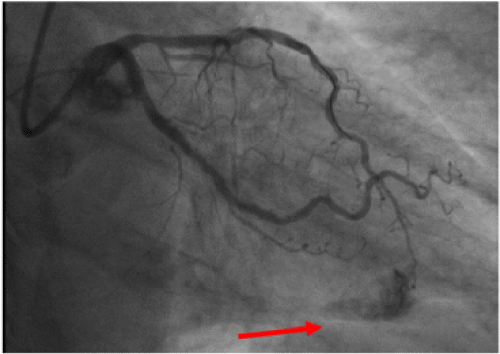
The immediate post-procedural coronary angiography demonstrated that we had achieved TIMI III grade coronary blood flow of the intervened vessel without any complications. However, patient’s clinical condition acutely deteriorated ~15 min. later with rapid fall of the systolic BP. The control coronary imaging revealed extravasation of contrast, indicative for perforation of the distal LAD segment (Figure 1). The Echo showed large pericardial effusion with cardiac tamponade.
Figure 1: Extravasation of contrast, indicative for perforation of the distal segment of the left anterior descending coronary artery.
Hemostasis and coronary embolization
Initially, we performed: 1) Percutaneous pericardial paracentesis, evacuating ~600 ml blood; 2) Venous application of protamine sulfate 50 mg; 3) Continuous (~15 min) intracoronary inflation of a balloon SeQuent 1.5/15 mm (Braun Ltd.), aiming to stop the intrapericardial hemorrhage. These measures had just temporary effect and patient’s condition did not improve: successful referral to the nearest cardiac surgery seemed very unlikely.
For this reason, we decided to improvise and to embolize the leaking site of LAD using an “embolic matrix”, prepared by ourselves from available materials. For the purpose, we used gelatin sponge Equispon® (Equimedical Ltd.), cutting it into miniature particles (<1 mm) and adding these particles to a mixture with contrast and saline.
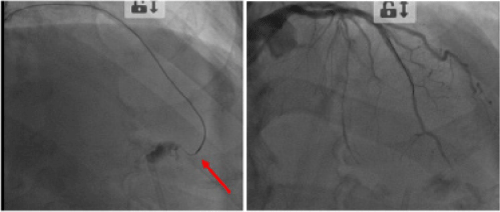
This “plugging” material was then delivered selectively at the site of perforation by microcatheter Corsair ® (Asahi Intecc Co., Ltd.). After two applications (1 ml each) we achieved complete “sealing” of the coronary perforation and cessation of the pericardial bleeding, confirmed by the repeated angiography and Echo (Figure 2).
Figure 2: Selective transcatheter application of the embolic matrix (to the left) led to complete sealing of the perforation (to the right).
The patient recovered completely and was discharged from our hospital 5 days later, asymptomatic and in good clinical condition. The pre-discharge hemoglobin was 116 g/L, troponin T 0.543 ng/ml, creatine kinase MB-fraction 28 U/L. The blood pressure at hospital discharge was 120/70 mm Hg and the control Echo showed 4-5 milimeters diffuse pericardial separation (non-significant pericardial effusion ~100 ml).
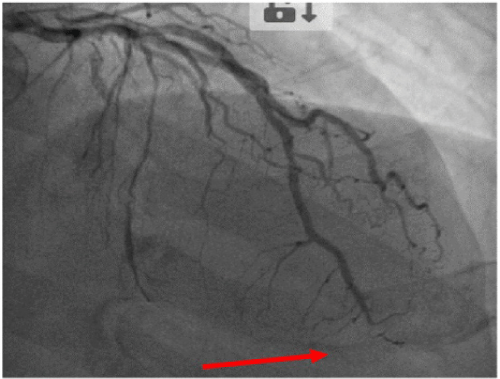
We performed control Echo and coronary angiography 3 month later: there was no residual pericardial effusion (the other Echo parameters were unchanged); the proximal LAD stent was found to be patent and there was subtotal occlusion of the apical segment of the same artery (Figure 3).
Discussion
We present a case of iatrogenic distal coronary perforation that occurred during revascularization of a complex, calcified LAD plaque, followed by development of cardiac tamponade and cardiogenic shock. We managed this life-threatening condition successfully using an improvised embolic plug, prepared from materials, largely available in most invasive centers. Our “bail-out” therapeutic approach turned out to be effective and safe, not causing any serious myocardial damage. We did not find in the literature other authors, who applied the same technique and for this reason we decided to present our case because it could be useful in similar cases of emergency.
Although considered relatively safe, percutaneous coronary interventions could at any moment be complicated by coronary perforation/rupture, particularly if coronary lesions are long and curved, tortuous, calcified, and/or revascularization of chronic occlusion is involved [1-5]. Such a coronary lesion was present in our patient. Other known risk factors for CAP are small vessel diameter, use of aggressive hydrophilic dilation coronary guide wires, high pressure and/or oversize balloons, etc. [3,8,9]. According to previous publications, coronary perforation leads to cardiac tamponade in 17% of all patients and the mortality rate is nowadays ~9% [3,5,10].
The standard treatment of CAP is cardiac surgery [9-12]. The development of new invasive techniques and devices led to reduction of CAPs, but also provided possibilities for their percutaneous, non-surgical treatment [8-12]. Unfortunately, most of the approved devices such as coated stents and coils are applicable only for proximal CAPs and 50-60% of perforations occur in the distal coronary segments, making the non-surgical treatment difficult [10-12]. The absence of a nearby cardiac surgery center makes the prognosis of these patients even worse [1,7,9]. The described “bail-out” method successfully ceased the bleeding from a distal LAD perforation, thus avoiding the need of emergent cardiac surgery. What is also important, is that the embolic “plug” was rapidly and easily prepared by the members of our team. The material used, was a gelatin sponge Equispon® (Equimedical Ltd.): in the routine clinical practice it is usually applied to bleeding wounds. The good results of our method were confirmed by the control Echo and coronary imaging, demonstrating complete cessation of pericardial bleeding, and by the fast clinical recovery of the patient.
Methods for management of distal CAPs, described by other authors are: microspheres, gel foam, two-component tissue glue, the Onyx system, thrombin or/and collagen injection, fat embolization, cyanoacrylate liquid glue, denatured alcohol, trisacryl gelatin microsphere, or polyvinyl alcohol particles and autologous blood clot embolization [3,8-12]. Most of them have to be ordered and provided in advance, i.e. they cannot be crafted from other available materials on site by the team in case of emergency [2,9-12]. Moreover, those that could be prepared such as fat tissue particles require more time and prior training for its preparation [9-12].
Conclusion
In summary, we consider that the described embolization technique with gelatin sponge particles is an effective and easy “bail-out” method of treatment of type V coronary vessels CAPs, provided other options are not available.
Implications to clinical practice
The method for coronary embolization, described by us can be used in the real clinical practice in cases of iatrogenic, distal coronary perforations/ruptures, particularly if other options (non-surgical or surgical) are not available at the moment, or if patient’s clinical condition requires fast solution due to rapid deterioration.
The used materials are largely available in most invasive centers and not expensive.The embolic matrix is easily and rapidly prepared, and deployed, not requiring specific skills.
This technique is effective and safe in terms of the short and long-term cessation of bleeding and the risk of post-procedural myocardial damage is low.
References
- Ajluni SC, Glazier S, Blankenship L, O’Neill WW, Safian RD. Perforation after percutaneous coronary interventions: clinical, angiographic and therapeutic observations. Cathet Cardiovasc Diagn 1994; 32: 206-212.
- Ramana RK, Arab D, Joyal D, Steen L, Cho L, et al. Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol. 2005;17: 603-605.
- Witzke CF, Martin-Herrero F, Clarke SC, Pomerantzev E, Palacios IF. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004; 16: 257-301.
- Milanova M H, Naydenov S N, Runev N M., Manov E I, Krastev P K. Analysis of prehospital care of patients with acute myocardial infarction in Bulgaria. Hong Kong Journal of Emergency Medicine 2018; 25: 196-201.
- Petrov I, Jorgova J, Runev N. The global revascularization – a modern approach in the treatment of patients with concomitant coronary, carotid and/or peripheral vascular disease. Bulgarian Cardiology 2003; 2: 27-30.
- Jorgova J, Petrov I, Trendafilova D, Dimitrov N, Runev N, et. al. Left main coronary artery stenting. Bulgarian Cardiology 2003; 3: 8-16.
- Trendafilova D, Jorgova J, Runev N. Bare metal stents as an acceptable option in diabetic patients. Comptes rendus de l`Academie bulgare des Sciences 2010; 63: 1681-1690.
- Shirakabe A, Takano H, Nakamura S, Kikuchi A, Sasaki A, et al. Coronary perforation during percutaneous coronary intervention. Int Heart J 2007; 48: 1-9.
- Javaid A, Buch AN, Satler LF, Javaid A, Buch AN, et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006; 98: 911-914.
- Al-Mukhaini M, Panduranga P, Sulaiman K, Riyami AA, Deeb M, et al. Coronary perforation and covered stents: an update and review. Heart Views. 2011; 12: 63-70.
- Yeo KK, Rogers JH, Laird JR. Use of stent grafts and coils in vessel rupture and perforation. J IntervCardiol 2008; 21: 86-99.
- Pershad A, Yarkoni A, Biglari D. Management of distal coronary perforations. J Invasive Cardiol. 2008; 20: 187-191.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.