Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Treatment of Hyponatremia with Acetazolamide in Cardiovascular patients: Possible Alternative Diuretic to Vasopressin Antagonists
- Hajime Kataoka
- School of Science, Hubei University of Technology, P. O. BOX 430068, P.R.China.

| Received | : | Oct 23, 2020 |
| Accepted | : | Nov 19, 2020 |
| Published Online | : | Nov 24, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Kataoka H. Treatment of Hyponatremia with Acetazolamide in Cardiovascular patients: Possible Alternative Diuretic to Vasopressin Antagonists. Ann Cardiol Vasc Med. 2020: 3(1); 1032.
Abstract
This study examined the effects of chloride-regaining diuretic acetazolamide (Diamox) on the serum sodium (Na) concentration in cardiovascular patients. Forty-three patients for whom Diamox was added as de-novo/add-on decongestion therapy for worsening heart failure (N= 21) or as modification therapy for hypochloremia (<100 mEq/L) in stable cardiovascular patients (N= 22) were retrospectively examined. Peripheral hematologic tests were performed at baseline, and at short- (≤10 days) and long-term (~60 days) time-points. In all the 43 study patients, an increased serum Na concentration correlated positively with an increased serum chloride concentration at both short- (N= 31; R2= 0.257, P= 0.0036) and long-term time-points (N=39; R2=0.634, P< 0.0001). Changes in serum Na concentration correlated negatively with serum Na concentration at baseline at both short- (N= 31; R2= 0.356, P= 0.0004) and long-term time-points (N= 39; R2= 0.321, P= 0.0002). When divided into two groups based on serum Na concentration before treatment, serum Na concentration in the group with hyponatremia (serum Na ≤ 135 mEq/L) increased from baseline to short-term time-point [N= 18; 132 ± 2.9 mEq/L (range 124 - 135 mEq/L) to 134 ± 3.2 mEq/L (range 128-141 mEq/L), P=0.01], and from baseline to long-term time-point [N= 21; 131 ± 4.1 mEq/L (range 120-135 mEq/L) to 136 ± 5.7 mEq/L (range 128-153 mEq/L), P= 0.0007]. Contrary, serum Na concentration in the group with normonatremia (serum Na>135 mEq/L) did not change from baseline to short-term time-point [N= 13; 141 ± 3.1 mEq/L (range 137-147 mEq/L) to 140 ± 3.1 mEq/L (range 137-147 mEq/L), P= 0.2], or from baseline to long-term time-point [N=18; 141 ± 2.4 mEq/L (range 137-145 mEq/L)] to 141 ± 1.7 mEq/L (range 138-144 mEq/L), P= 0.77]. In conclusion, acetazolamide has favorable effects on correction of hyponatremia, suggesting that acetazolamide could be an alternative diuretic to vasopressin antagonists in some cardiovascular patients.
Introduction
Recent studies demonstrated that chloride is a key electrolyte regulating plasma volume during worsening Heart Failure (HF) [1] and its recovery following treatment with conventional diuretics [2], leading to the “chloride theory” for HF pathophysiology [3,4] and a diuretic strategy based on modulating the serum chloride concentration in decompensated HF patients [2,4]. According to the “chloride theory” for HF pathophysiology [4], manipulation of the serum chloride concentration using the carbonic anhydrase inhibitor acetazolamide could be an attractive HF treatment, as described in recent reports [5,6]. My experiences described in recent case report [7] revealed the potential of the classic diuretic acetazolamide to correct hyponatremia. Except for a few early case presentations [8,9] describing such a phenomenon, few studies have evaluated the effects of acetazolamide on hyponatremia. The present study, therefore, aimed to clarify the short- and long-term effects of acetazolamide on changes in the serum sodium (Na) concentration, particularly hyponatremia, in cardiovascular patients.
Materials and methods
Study design
This retrospective single-center observational study analyzed clinical data derived from an intentional study to investigate the effects of acetazolamide (Diamox) on the serum chloride concentration and other hematologic values in cardiovascular patients over short- and long-term time periods. The total study period was from February 2016 to October 2018, during which Diamox was prescribed and its efficacy to treat acutely decompensated HF patients or correct low serum chloride concentrations (<100 mEq/L) in stable cardiovascular patients was evaluated. The ethics committee at Nishida Hospital approved the study protocol.
Study patients
Among 95 cardiovascular patients, after excluding 52 patients with replacement/combined usage of Diamox as well as other diuretic(s), the present study retrospectively examined 43 consecutive patients for whom Diamox was added as de-novo/add-on decongestion therapy in acutely worsening HF patients or as modification therapy for a low serum chloride concentration (<100 mEq/L) in stable cardiovascular patients with or without chronic HF. In the 43 patients enrolled in the present investigation, background use of diuretics other than Diamox was absent (de-novo use of Diamox) or continued at a constant dosage (add-on use of Diamox) at least seven days before the initiation of Diamox and throughout the period of evaluation of Diamox treatment to short- (≤10 days) or long-term (~60 days) time-points.
Treatment protocol and data collection
In acutely decompensated HF patients, HF-related physical sign(s), body weight, and serum b-type natriuretic peptide levels were checked at the initial presentation and at appropriate intervals during the study period. Worsening HF was determined based on the appearance of at least one of the following HF-related signs during follow-up, whether or not changes in symptoms occurred: physical signs of congestion (peripheral edema, pulmonary crackles, the third heart sound) and/or pleural effusion on ultrasound [2]. At the follow-up examination, response to Diamox treatment for worsening HF was determined by weight reduction and resolution of HF-related signs.
A low-dose of Diamox (250-750 mg/d) was prescribed for each patient once or twice a day. The dose of Diamox was titrated up or down at my discretion based on blood test results and the patient’s condition including HF status, if present, peripheral hematologic tests were performed at baseline, and at short- (≤10 days) and long-term (~60 days) time-points. If multiple examinations were performed during the study period, the latest available examination at the end of each time-point was selected for analysis.
Hyponatremia and hypochloremia were defined as a serum Na concentration of ≤135 mEq/L [10] and a serum chloride concentration of ≤96 mEq/L [11,12] respectively.
Statistical analysis
All statistical analyses were performed using commercially available statistical software GraphPad Prism 4 (San Diego, CA). All data are expressed as a mean ± SD for continuous data and percentage for categorical data. Paired and unpaired t tests for continuous data and Fisher’s exact test for categorical data were used for two-group comparisons. Correlations between two parameters were analyzed using Pearson’s correlation, and demonstrated using scatter plots. All tests were 2-tailed, and P value < 0.05 was considered statistically significant.
Results
The clinical characteristics of the 43 patients at study entry are shown in Table 1. Mean patient age was 84.8 ± 7.8 years, and 35% were male. The primary cause of cardiovascular disease varied, with mean left ventricular ejection fraction in 58.4 ± 14.0%, and atrial fibrillation in 15 (35%) patients. Details of Diamox treatment for study patients are presented in Table 2. Diamox was prescribed for treatment of acutely decompensated HF in 21 study patients (de-novo usage in 2), and for modification of low serum chloride concentration in 22 study patients (de-novo usage in 7). The daily dose of Diamox was greater for the patients treated for acute HF than for patients treated to modify hypochloremia (363 ± 158 mg/d vs 239 ± 101 mg/d, P= 0.0036).
In acutely decompensated HF patients, Diamox treatment for 27 ± 14 days (range, 7 - 60 days) resolved the HF-related signs (≧1) and/or body weight reduction (≧1kg) in 17 of 21 patients (81%). Body weight (n = 17) decreased from 45.2 ± 10.3 kg at baseline to 43.3 ± 10.3 kg after treatment (P < 0.0001) with a mean reduction of -1.89 ± 1.39 kg (range, -4.7 to 0.7 kg). Serum BNP levels decreased in 17 of 19 patients (89%) after treatment; the serum log BNP level (n= 19) decreased from 2.40 ± 0.29 pg/mL at baseline to 2.13 ± 0.40 pg/mL after treatment (P= 0.0004), with a mean reduction of -0.26 ± 0.27 pg/mL. Among cardiovascular patients assigned to the modification treatment, body weight did not change in a total of measured 17 patients (from 48.7 ± 9.90 kg at baseline to 48.2 ± 9.42 kg after treatment [P= 0.16] with a mean reduction of -0.51 ± 1.41 kg; range, -3.0 to 1.7 kg) after Diamox treatment for 32.5 ± 15.4 days (range, 10 - 57 days), though weight reduction ≧1kg (range, -1kg to -3kg) was observed in 5 patients.
Changes in blood test values during the short- and long-term periods in all 43 patients are shown in Table 3. Compared with the baseline levels, the serum chloride concentration increased in the short- and long-term observation periods. The serum potassium concentration constantly decreased throughout both the short- and long-term study periods. The blood urea nitrogen, serum creatinine, and uric acid concentrations did not change during the study period.
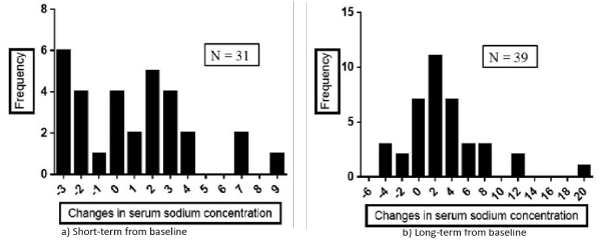
The mean serum Na concentration in the overall patients after acetazolamide treatment did not change during the short-term observational period, and only slightly increased during the long-term observation period (Table 3), but the changes were heterogeneously and widely distributed as shown in Figure 1. Namely, the amount of change in the serum Na concentration during the short-term period (n= 31; mean= 0.90 ± 3.23, range, -3 to +9; Figure 1, left panel) was randomly distributed and the change in the serum Na concentration was equal to 0 in 4 patients (13%), greater than 0 in 16 (52%), and less than 0 in 11 (35%). During the long-term period (n= 39; 2.51 ± 4.62; range -5 to +19; Figure 1, right panel), the amount of change in the Na concentration became more normally, but still widely distributed, and the change was equal to 0 in 7 patients (18%), greater than 0 in 27 (69%), and less than 0 in 5 patients (13%).
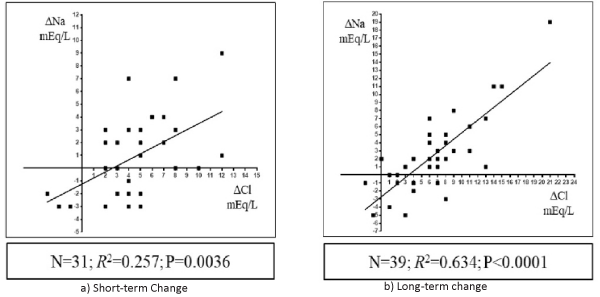
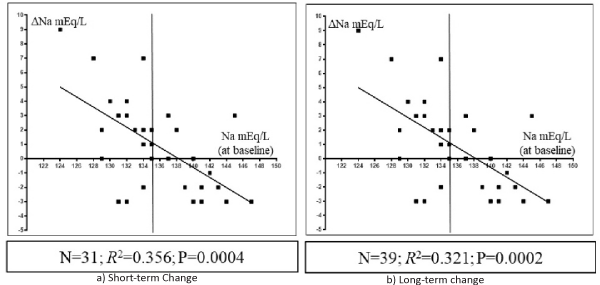
In all 43 patients, the increase in the serum Na concentration correlated positively with the increase in the serum chloride concentration from baseline at both short- (n= 31; R2= 0.257, P= 0.0036; Figure 2, left panel) and long-term time-points (n = 39; R2=0.634, P < 0.0001; Figure 2, right panel) after Diamox treatment. Changes in the serum Na concentration correlated negatively with the baseline serum Na concentration at both short- (n= 31; R2= 0.356, P= 0.0004; Figure 3, left panel) and long-term time-points (n = 39; R2= 0.321, P= 0.0002; Figure 3, right panel).
Blood test values after Diamox treatment were compared between the different baseline serum Na concentrations (Table 4). When divided into two groups, the serum Na concentration in the group with hyponatremia (serum Na ≤ 135 mEq/L) significantly increased from baseline to short-term time-points [n = 18; from 132 ± 2.9 mEq/L (range 124-135 mEq/L) to 134 ± 3.2 mEq/L (range 128-141 mEq/L), P= 0.01], and from baseline to the long-term time-points [n= 21; from 131 ± 4.1 mEq/L (range 120-135 mEq/L) to 136 ± 5.7 mEq/L (range 128-153 mEq/L), P= 0.0007]. In contrast, the serum Na concentration in the group with normonatremia (serum Na > 135 mEq/L) did not change from baseline to the short-term time-point [n= 13; from 141 ± 3.1 mEq/L (range 137-147 mEq/L) to 140 ± 3.1 mEq/L (range 137-147 mEq/L), P = 0.2], and baseline to the long-term time-point [n= 18; from 141 ± 2.4 mEq/L (range 137-145 mEq/L)] to 141 ± 1.7 mEq/L (range 138-144 mEq/L), P= 0.77].
Figure 1: Distribution of the amount of change in the serum sodium (Na) concentration from baseline to the short-term (a) and long-term (b) time-points.
Figure 2: Scatterplots graph of the relationship between the changes in the serum chloride (Cl) concentration (x-axis) from baseline to short-term (a) or long-term (b) time-points and the change in the serum sodium (Na) concentration (y-axis).
Figure 3: Scatterplots showing the relationship between the serum sodium (Na) concentration at baseline (x-axis) and the change in the serum Na concentration (y-axis) from baseline to short-term (a) or long-term (b) time-points. Vertical line across the Na concentration of 135 mEq/L at the x-axis is shown.
Table 2: Usage of acetazolamide for treatment of acutely decompensated heart failure or correction of hypochloremia.
Table 3: Changes in peripheral hematologic test and blood biochemistry in a total of the study patients (n = 43) under acetazolamide de-novo/add-on treatment.
Table 4: Comparison of effects of acetazolamide on serum sodium (Na) concentration between cardiovascular patients with or without hyponatremia.
Discussion
The findings of the present study demonstrated favorable effects of acetazolamide for modulating disturbances in the serum Na concentration in cardiovascular patients. Interestingly, acetazolamide treatment had no significant effect on the serum Na concentration in normonatremic patients, but favorably corrected the disturbance of the serum Na concentration in hyponatremic patients, in which the lower the serum Na concentration before treatment, the greater the increase in the serum Na concentration after acetazolamide treatment.
Re-appraisal of acetazolamide in the mainstay of HF treatment
Early studies demonstrated that the carbonic anhydrase inhibitor acetazolamide might be effective for patients with refractory HF [8,9,13-16]. This diuretic has long been outside of the mainstay decongestive treatment for worsening HF because of the subsequent development of more potent loop and thiazide diuretics. Several recent studies, however, re-evaluated the effects of acetazolamide and suggested its advantageous effects for treatment of refractory HF status [17-22]. Carbonic anhydrases play important roles in acid-base transport in the proximal tubule and collecting duct [23,24]. Inhibition of carbonic anhydrase activity in the proximal tubule by acetazolamide blocks apical Na+/H+ exchanger activity and decreases Na and bicarbonate reabsorption [23,24]. Although the diuretic and natriuretic capacity of acetazolamide by itself might be poor, the potential effectiveness of this agent on refractory HF involves a synergic diuretic effect in combination with a loop or a thiazide diuretic through: 1) sequential blockade of Na reabsorption in the proximal tubules by acetazolamide, and in both Henle’s loop and distal tubules by both loop and thiazide diuretics [18,23] or 2) enhancement of the diuretic effect of thiazides by urine alkalization [25]. Furthermore, acetazolamide could improve thiazide-like diuretic efficacy, as it potently downregulates pendrin expression in the distal nephrons [25, 26]. A large randomized, double-blind, placebo-controlled study, such as the ongoing OveRLoad (ADVOR) trial [27] is expected to provide real-world clinical evidence for the beneficial contribution of acetazolamide to HF management.
Effects of acetazolamide on the serum Na concentration
Effects of acetazolamide on serum chloride, potassium, or bicarbonate are clinically well investigated [8,13,14,17]. But there is an unexpectedly scant amount of clinical data and, if available at all, only a brief description of its effects on the serum Na concentration [8,9,13-15,17].
Acetazolamide has unique but critical diuretic actions: a “non-reabsorbable anion-like effect”, that results in the excretion of bicarbonate into the urinary tubules with interchangeable absorption of filtrated chloride into the blood, and concurrent excretion of potassium into the urine [5,8,13,14,18,19,23,24]. An earlier study by Relman et al [14] evaluated the acute effects (3-12 days) of acetazolamide treatment on serum solutes in 26 patients with severe HF (men, 73%; age, 56.7 ± 12.4 years). Re-evaluation of their data by Kataoka [5,7] revealed that the serum Na concentration was unchanged or increased after treatment with acetazolamide in 73% of 24 evaluations. The results of the present study demonstrated not only a short-term effect of acetazolamide on the serum Na concentration, similar to the findings reported by Relman et al [5,7,14], but also persistent long-term effects lasting at least ~ 60 days. Interestingly, as shown in the present study, the modulating effects of this diuretic agent in cardiovascular patients were favorably characterized by modulation of the serum Na concentration only in those with hyponatremia, and not in those with normonatremia.
Possible mechanism of acetazolamide for correcting hyponatremia
The mechanisms responsible for the effects of acetazolamide to modulate hyponatremia are assumed to be very complex because electrolyte abnormalities may be multifactorial and interrelated, resulting from neurohormonal activation, renal dysfunction, medications, and dietary intake [28-30]. Nonetheless, when considering the mechanisms underlying the effects of acetazolamide to correct hyponatremia, there are at least a few possibilities regarding its actions to increase the serum Na concentration, despite its natriuretic action, as follows: 1) aquaretic effect of this agent, i.e., an increase in the free water clearance in the urinary tubules, 2) mobilization of Na from other spaces into the vascular space, and 3) a combination of these actions. Early studies [8,9] described an excess rate of water diuresis in relation to the urinary electrolytes in several cases, but the exact mechanism for this phenomenon is unclear. Thus, it is worthwhile to examine the effect of acetazolamide on arginine vasopressin [31] or renal aquaporin receptors [32]. To address this issue, indeed, a recent experimental study indicated an effect of acetazolamide on aquaporin-1 protein expression [33], through which water balance might be modulated [32]. Another experimental study reported that increased diuresis in rats treated with a combination of acetazolamide and thiazide was associated with a significant reduction in urine osmolality and reduced membrane localization of aquaporin-2 [25].
The present study revealed that an increase in the serum Na concentration positively correlated with an increase in the serum chloride concentration under acetazolamide treatment. This observation suggests that an accumulation of anionic (negatively-charged) chloride in the vascular space would electrically attract cationic (positively-charged) Na electrolytes from the interstitial space into the vascular space because Na is not distributed in the body solely as a free cation, but it is also bound to large interstitial glycosaminoglycan networks in different tissues, which may have an important regulatory function for the serum Na concentration or quantity by transferring Na from the interstitial space into the blood [34-36].
According to these possible mechanisms mentioned above, carbonic anhydrase acetazolamide has a potential for correcting hyponatremia by increasing the free water clearance in the urinary tubules and mobilizing Na from the interstitial space into the vascular space. Exact mechanisms for correcting disturbances in the serum Na concentration by carbonic anhydrase acetazolamide, however, require further exploration.
Study limitations
This study has some limitations. First, this study was performed in a relatively small number of patients, was a single-center observational study, and should thus be considered to be hypothesis-generating. Second, the present study is a sub-analysis of the data collected from a study primarily investigating the effects of acetazolamide on the serum chloride concentration [37]. So further investigations designed to determine the effects of acetazolamide on hyponatremic patients are needed. Finally, the sole effect of acetazolamide on the serum Na concentration is unclear in the present clinical study because most of the study patients were in the group with add-on usage of acetazolamide to other conventional diuretics.
Conclusions
The carbonic anhydrase inhibitor acetazolamide, the oldest diuretic among commercially available diuretics, exhibited favorable effects for modulating hyponatremia in cardiovascular patients, indicating that acetazolamide could be an alternative diuretic to vasopressin antagonists for some proportion of HF patients with hyponatremia. Its effect to enhance the serum Na concentration occurs promptly within 10 days and persists for at least ~ 60 days. Large randomized studies are required to re-evaluate the efficiency of this forgotten, but useful agent for the treatment of HF and its potential for correcting hyponatremia. Importantly, the efficiency of this diuretic agent to correct hyponatremia in comparison with vasopressin antagonists would presumably be modest [38-40]; therefore, worsening hyponatremia even with the use of this agent should be carefully evaluated.
References
- Kataoka H. Vascular expansion during worsening of heart failure: effects on clinical features and its determinants. Int J Cardiol. 2017; 230: 556-561.
- Kataoka H. Biochemical determinants of changes in plasma volume after decongestion therapy for worsening heart failure. J Card Fail. 2019; 25: 213-217.
- Kataoka H. Proposal for heart failure progression based on the “chloride theory”: worsening heart failure with increased vs. non-increased serum chloride concentration. ESC Heart Fail. 2017; 4: 623-631.
- Kataoka H. The “chloride theory”, a unifying hypothesis for renal handling and body fluid distribution in heart failure pathophysiology. Med Hypotheses. 2017; 104: 170-173.
- Kataoka H. Treatment of hypochloremia with acetazolamide in an advanced heart failure patient and importance of monitoring urinary electrolytes. J Card Cases. 2018; 17: 80-84.
- Kataoka H. Comparison of changes in the plasma volume and renal function between acetazolamide vs. conventional diuretics: understanding their mechanical differences according to the chloride theory. Eur Heart J. 2018; 39: 40-41 (abstract).
- Kataoka H. Vasopressin antagonist-like effect of acetazolamide in a heart failure patient: A case report. Eur Heart J Case Rep. 2018; 2: 1-5.
- Counihan TB, Evans BM, Milne MD. Observations on the pharmacology of the carbonic anhydrase inhibitor “Diamox”. Clin Sci. 1954; 13: 583-598.
- Braveman WS, Rubin AL. Treatment of the low-salt syndrome in congestive heart failure by the controlled use of mercurial diuretics. Circulation. 1956; 13: 655-663.
- Ghali JK, Tam SW. The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists. J Card Fail. 2010; 16: 419-431.
- Testani JM, Hanberg JS, Arroyo JP, Brisco MA, ter Maaten JM, et al. Hypochloremia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016; 18: 660-668.
- Hanberg JS, Rao V, ter Maaten JM, Laur O, Brisco MA, et al. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016; 9: e003180.
- Friedberg C, Taymor R, Minor JB, Halpern M. The use of Diamox, a carbonic anhydrase inhibitor, as an oral diuretic in patients with congestive heart failure. N Engl J Med. 1953; 248: 883-889.
- Relman AS, Leaf A, Schwartz WB. Oral administration of a potent carbonic anhydrase inhibitor (“Diamox”): II. Its use as a diuretic in patients with severe congestive heart failure. N Engl J Med. 1954; 250: 800-804.
- Leaf A, Schwartz WB, Relman AS. Oral administration of a potent carbonic anhydrase inhibitor (“Diamox”): I. changes in electrolyte and acid-base balance. N Engl J Med. 1954; 250:759-764.
- Rubin AL, Thompson HG Jr, Braveman WS, Luckey EH. The management of refractory edema in heart failure. Ann Intern Med. 1955; 42: 358-368.
- Khan MI. Treatment of refractory congestive heart failure and normokalemic hypochloremic alkalosis with acetazolamide and spironolactone. Can Med Assoc J. 1980; 123: 883-887.
- Knauf H, Mutschler E. Sequential nephron blockade breaks resistance to diuretics in edematous states. J Cardiovasc Pharmacol. 1997; 29: 367-372.
- Caramelo C, Albalate M, Tejedor A, Alcázar R, Baldoví S, et al. Actuality of the use of acetazolamide as a diuretic: usefulness in refractory edema and in aldosterone-antagonist-related hyperkalemia. Nefrologia. 2008; 28:234-238
- Kassamali R, Sica DA. Acetazolamide: a forgotten diuretic agent. Cardiol in Rev. 2011; 19: 276-278.
- Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015; 70: 265-273.
- Imiela T, Budaj A. Acetazolamide as add-on diuretic therapy in exacerbations of chronic heart failure: a pilot study. Clin Drug Investg. 2017; 37: 1175-1181.
- Seely JF, Dirks JH. Site of action of diuretic drugs. Kidney Int. 1977; 11: 1-8.
- Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007; 71: 103-115.
- Zahedi K, Barone S, Xu J, Soleimani M. Potentiation of the effect of thiazide derivatives by carbonic anhydrase inhibitors: molecular mechanisms and potential clinical implications. PLoS One. 2013; 8: e79327.
- Amlal H, Soleimani M. Pendrin as a novel target for diuretic therapy. Cell Physiol Biochem. 2011; 28: 521-526.
- Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail. 2018; 20: 1591-1600.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007; 120: S1-S21.
- Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WHW, et al. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol. 2015; 65: 480-492.
- Grodin JL. Pharmacologic approaches to electrolyte abnormalities in heart failure. Curr Heart Fail Rep. 2016; 13: 181-189.
- Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006; 290: F273-F278.
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996; 58: 619-648.
- Xiang Y, Ma B, Li T, Gao JW, Yu HM, Li XJ. Acetazolamide inhibits aquaporin-1 protein expression and angiogenesis. Acta Pharmacol Sin. 2004; 25: 812-816.
- Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015; 65: 378-388.
- Nijst P, Olinevich M, Hilkens P, Martens P, Dupont M, et al. Dermal interstitial alterations in patients with heart failure and reduced ejection fraction: a potential contributor to fluid accumulation? Circ Heart Fail. 2018; 11: e004763.
- Wiig H. Regulation of fluid volume from the outside: a role of glycosaminoglycans in the skin interstitium? Circ Heart Fail. 2018; 11: e005135.
- Kataoka H. Acetazolamide as a potent chloride-regaining diuretic: short- and long-term effects, and its pharmacologic role under the ‘chloride theory’ for heart failure pathophysiology. Heart Vessels, 2019; 34: 1952-1960.
- Gheorghiade M, Gattis WA, O’Connor CM, Adams KF Jr, Elkayam U, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004; 291:1963-1971.
- Aronson D, Verbalis JG, Mueller M, Krum H, on behalf of the DILIPO investigators Short- and long-term treatment of dilutional hyponatraemia with satavaptan, a selective arginine vasopressin V2-receptor antagonist: the DILIPO study. Eur J Heart Fail. 2011; 13: 327-336.
- Kataoka H, Yamasaki Y. Strategy for monitoring decompensated heart failure treated by an oral vasopressin antagonist with special reference to the role of serum chloride: a case report. J Card Cases. 2016; 14: 185-188.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.