Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
The Influence of Cancer Therapy on Cardiovascular Events
- Humma H Khan;
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.
- Shazma I Khan*;
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.
- Zainab S Dar;
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.
- Hemlata Limbu;
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.
- Ralitsa Pancheva;
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.
- Blagovest Stoimenov
- Cardiology Clinic, Department of Internal Diseases “Kirkovich”, University Hospital Alexandrovska, Sofia, Bulgaria.

| Received | : | Feb 04, 2021 |
| Accepted | : | Mar 05, 2021 |
| Published Online | : | Mar 10, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Khan HH, Khan SI, Dar ZS, Limbu H, Pancheva R, et al. The Influence of Cancer Therapy on Cardiovascular Events. Ann Cardiol Vasc Med. 2021: 4(1); 1045.
Abstract
Introduction: Heart disease following cancer treatment may be directly linked to cardiovascular damage which may have been induced by chemotherapy treatment or accelerate the disease process ofatherosclerosis itself, including cancer treatment-related cardiovascular risk factors such as hypertension, diabetes mellitus, and dyslipidemia compared with age-matched counterparts with no cancer history [4]. Therefore, our study aimed to assess the relationship between breast and prostate cancer treated patients and their current cardiovascular presentations.
Method: A total of 100 patient’s medical history were identified with breast cancer or prostate cancer and the relationship between cancer treated patients and patients with no cancer was assessed with the cardiovascular parameters. Statistical analysis was conducted using IBM®SPSS® Statistical software version 22. Fischer-Exact test (p ≤ 0.01) significance test with two-sided calculations were performed.
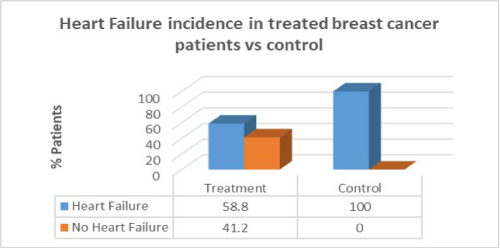
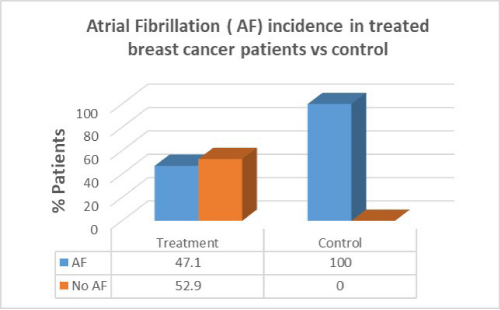
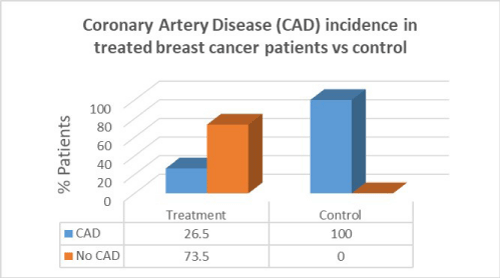
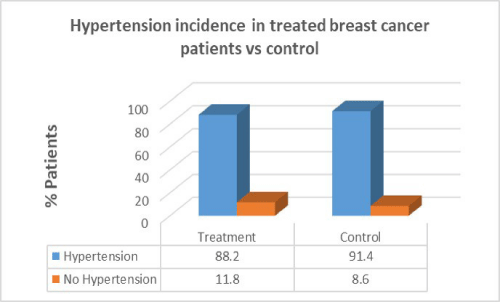
Results: Women with breast cancer who had previous cancer treatment had presented with a higher incidence of heart failure (58.8%) compared to women with no heart failure in the same group (41.2%). Women with breast cancer who had previous cancer treatment presented with lower incidence of atrial fibrillation (AF) (47.1%) compared with women with no AF in the same group (52.9%). Women with breast cancer who had previous cancer treatment presented with significantly lower incidence of coronary artery disease (CAD) (26.5%) compared with females with no CAD in the same group (73.5%). Women with breast cancer who had previous cancer treatment presented with significantly higher incidence of hypertension (88.2%) than in women with no hypertension in the same group (11.8%). women with breast cancer who had previous cancer treatment presented with lower incidence of valvular disease (38.2%) than in women with no valvular disease in the same group (61.8%).
Men with prostate cancer who had previous cancer treatment presented with significantly higher incidence of heart failure (83.3%) than in men with no heart failure in the same group (16.7%). Men with prostate cancer who had previous cancer treatment presented with lower incidence of AF (25%) than in men with no AF in the same group (75%).
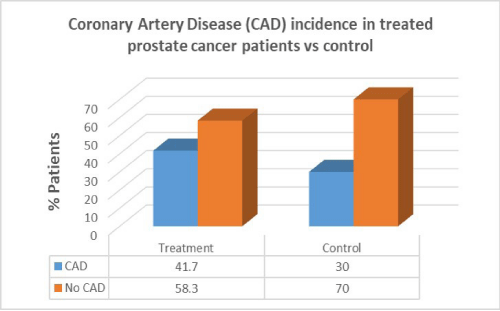
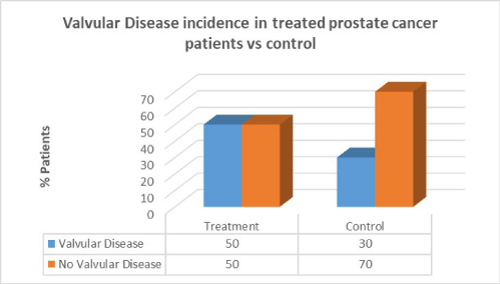
Men with prostate cancer who had previous cancer treatment presented with significantly higher incidence of hypertension (91.7%) than in men with no hypertension in the same group (8.3%). Men with prostate cancer who had previous cancer treatment presented with lower incidence of CAD (41.7%) than in men with no CAD in the same group (58.3%). Men with prostate cancer who had previous cancer treatment presented with the same incidence of valvular disease (50%) than in men with no valvular disease in the same group (50%).
Discussion: This study demonstrates that there is no correlation between breast cancer patients with prior cancer treatment to any of the cardiovascular parameters looked at in this study. The same results were achieved with patients with prostate cancer however, the study did show a correlation between treated prostate cancer patients to heart failure.
Objective: the objective of this study is to assess the effects of cancer treatment on cardiovascular parameters (such as heart failure, hypertension, atrial fibrillation, valvular disease and coronary artery disease) in patients with breast cancer and prostate cancer.
Introduction
Advances in early detection and treatment have significantly improved the 5-year disease-specific survival rates for the 10 most common malignancies. Improvement in treatment modalities, including radiotherapy and systemic therapies, has led to better prognosis for patients with malignancies. Unfortunately, they may also induce late effects including an increased risk of Cardiovascular Disease (CVD) in long-term survivors [3,4], specifically, cancer survivors living at least 5 years beyond diagnosis have a 1.3- to 3.6-fold increased risk of cardiovascular-specific mortality and a 1.7- to 18.5-fold increased incidence of CVD risk factors such as hypertension, diabetes mellitus, and dyslipidemia compared with age-matched counterparts with no cancer history [4]. Studies have also shown that patients with one or more cardiovascular risk factors are more likely to experience a cardiac event when exposed to cancer therapy [3].
Heart disease following cancer treatment may be the result of direct cardiovascular damage caused by the treatment itself or of accelerated atherosclerosis due to cancer treatment-related cardiovascular risk factors [5]. In the general population, CVDs are major causes of morbidity [5]and mortality, accounting for 30–50% of all deaths in most developed countries. Because of this high background rate, even a minor increase in risk of CVD will have an important impact on morbidity and mortality.
Radiotherapy for early-stage breast cancer can reduce the rates of recurrence and of death from breast cancer. However, long-term follow-up in some trials has shown that radiotherapy can also increase the risk of ischemic heart disease, presumably through incidental irradiation of the heart [1,2].
Survivors of prostate cancer have an increased risk for heart disease. It is the most common noncancer cause of death for men with prostate cancer. Early detection, effective treatment, and the disease’s slow progression have meant that survivors of prostate cancer will die from something other than cancer. Cardiovascular disease is particularly a concern for men who received Androgen-Deprivation Therapy (ADT) to treat their prostate cancer. While ADT therapy is of great benefit to many patients with prostate cancer, it may also increase the risk of developing diabetes or having a heart attack or stroke [8].
Newly developed targeted therapy can exert off-target effects causing hypertension, thromboembolism, QT-prolongation and atrial fibrillation. The variety of cardiovascular side-effects from systemic cancer therapies is diverse and includes the induction of cardiac dysfunction, myocardial ischemia, arrhythmias, thromboembolism, arterial and pulmonary hypertension, peripheral arterial occlusive disease and pleural effusion. Cardiotoxicity following systemic treatment is typically associated with loss of myocardial mass, leading to progressive cardiac remodeling and dysfunction. Patients experiencing cardiotoxicity develop Heart Failure (HF) months to years after the initial cancer therapy and have a severely impaired cardiovascular prognosis [6].
Radiation therapy often accelerates atherosclerosis. Furthermore, radiation can damage the heart valves, the conduction system, and pericardium that may take years to manifest clinically. Management of pericardial disease in cancer patients also posed clinical challenges (1). Radiation therapy affects all cardiac structures including the pericardium, epicardial and microvascular circulation, conduction system, and the myocardium. Patients can present with acute pericarditis immediately following radiation therapy or chronic pericarditis decades after radiation therapy. Valvular heart disease and coronary artery disease usually presents 5 to 10 years after radiation therapy [7].
Hypertension (HTN) is the most common cardiovascular comorbidity reported in cancer registries with a prevalence of 37%. Early diagnosis and treatment are essential because HTN is a major risk factor for the development of chemotherapy-induced cardiotoxicity. In addition, suboptimal blood pressure control may lead to premature discontinuation of chemotherapy, thus affecting cancer therapy directly [5].
Cardiologists are more likely than oncologists to recommend early referral of cancer patients to a cardio-oncology clinic. Therefore, there has been a shift towards a multidisciplinary approach for the optimal patient specific cancer treatment which has led to a new dynamic approach with a clear focus to prevent short term cardiovascular harm and promote long term cardiovascular health after the cancer treatment [3].
Therefore, our study aimed to assess the relationship between breast and prostate cancer treated patients and their current cardiovascular presentations.
Methods
Patient records from the department of Cardiology, Alexandrovska Hospital, Sofia, Bulgaria were used to obtain data of all the patients assessed in the study. A total of 100 patient’s medical history were identified: 34 women with breast cancer and anticancer treatment, 35 women with no cancer, 11 men with prostate cancer and anticancer treatment and 20 men with no cancer diagnosis currently or in the past (‘no cancer’ patients were used as the control).
The hospital records of these patients were used to determine which individuals were diagnosed with Heart Failure (HF), Atrial Fibrillation (AF), hypertension, valvular disease and Coronary Vascular Disease (CAD). Valvular disease included patients with mitral stenosis, mitral insufficiency, aortic stenosis, aortic insufficiency and tricuspid insufficiency. CAD included myocardial infarction, stable angina, unstable angina and ischaemic heart disease.
The relationship between cancer treated patients and patients with no cancer was assessed with the cardiovascular parameters. Statistical analysis was conducted using IBM®SPSS® Statistical software version 22. Fischer-Exact test (p ≤ 0.01) significance test with two-sided calculations were performed.
Results
Women with breast cancer who had previous cancer treatment presented with a higher incidence of heart failure (58.8%) than in women with no heart failure in the same group (41.2%). However, in the control group there was a greater percentage of heart failure patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of heart failure (p=0.09).
Women with breast cancer who had previous cancer treatment presented with lower incidence of Atrial Fibrillation (AF) (47.1%) than in women with no AF in the same group (52.9%). In the control group there was a greater percentage of AF patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of AF (p=0.07).
Women with breast cancer who had previous cancer treatment presented with significantly lower incidence of Coronary Artery Disease (CAD) (26.5%) than in women with no CAD in the same group (73.5%). In the control group there was a greater percentage of CAD patients compared to patients who had cancer treatment. There was, however, a correlation between cancer treatment and presentation of CAD (p=0.001).
Women with breast cancer who had previous cancer treatment presented with significantly higher incidence of hypertension (88.2%) than in women with no hypertension in the same group (11.8%). In the control group there was a greater percentage of hypertension patients (91.4%) compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of hypertension (p=0.710).
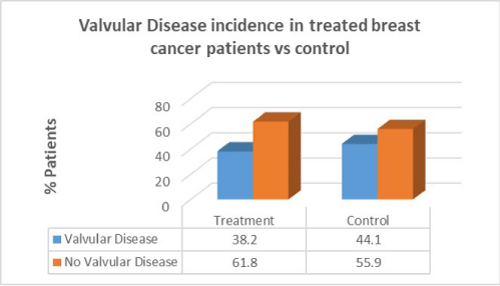
Women with breast cancer who had previous cancer treatment presented with lower incidence of valvular disease (38.2%) than in women with no valvular disease in the same group (61.8%). In the control group there was a greater percentage of valvular disease patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of valvular disease (p=0.8).
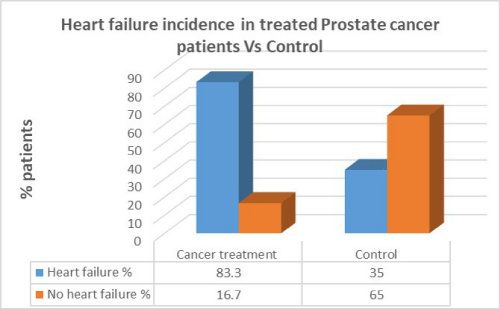
Men with prostate cancer who had previous cancer treatment presented with significantly higher incidence of heart failure (83.3%) than in men with no heart failure in the same group (16.7%). In the control group there was a lesser percentage of heart failure patients compared to patients who had cancer treatment. There was a correlation between cancer treatment and presentation of heart failure in prostate cancer (p=0.01).
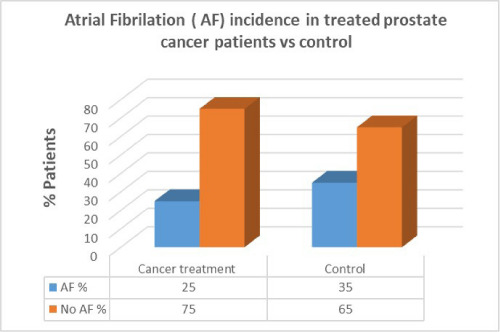
Men with prostate cancer who had previous cancer treatment presented with lower incidence of AF (25%) than in men with no AF in the same group (75%). In the control group there was a higher percentage of AF patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of AF in prostate cancer (p=0.7).
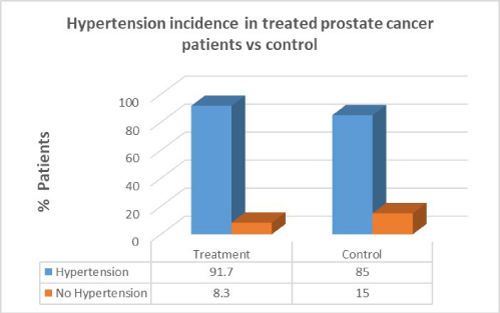
Men with prostate cancer who had previous cancer treatment presented with significantly higher incidence of hypertension (91.7%) than in men with no hypertension in the same group (8.3%). In the control group there was a lower percentage of hypertension patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of hypertension in prostate cancer (p=1.0).
Men with prostate cancer who had previous cancer treatment presented with lower incidence of CAD (41.7%) than in men with no CAD in the same group (58.3%). In the control group there was a lower percentage of CAD patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of CAD in prostate cancer (p=0.7).
Men with prostate cancer who had previous cancer treatment presented with the same incidence of valvular disease (50%) than in men with no valvular disease in the same group (50%). In the control group there was a lower percentage of valvular disease patients compared to patients who had cancer treatment. There was no correlation between cancer treatment and presentation of valvular disease in prostate cancer (p=0.2).
Discussion
This study demonstrates that there is no correlation between breast cancer patients with prior cancer treatment to any of the cardiovascular parameters looked at in this study. The same results were achieved with patients with prostate cancer however, the study did show a correlation between treated prostate cancer patients to heart failure.
Increase in mortality and morbidity from heart disease has also been reported after radiotherapy for Breast Cancer (BC), especially after some of the radiotherapy techniques that were used in the past [9,10].
In a recent publication 963 women who experienced a major coronary event after radiotherapy for BC between 1958 and 2001 in Sweden and Denmark were compared with 1205 control women who were also irradiated for BC but did not have a major coronary event [11]. An increased risk of major coronary events was observed that started within the first 5 years after radiotherapy and continued into the third decade. The major coronary event rate increased linearly with the mean dose to the heart by 7.4% per Gy (95% confidence interval, 2.9–14.5; P < 0.001), with no apparent threshold.
Classical risk factors for coronary artery disease also influence the risk of radiation-related CVDs. Higher risks of developing CVDs following exposure of the heart to radiation have been observed in patients with classical risk factors for CVDs. For example, in a large study in 10-year survivors of BC, smoking and radiotherapy together were associated with an even more than additive effect on risk of MI [10].
Data on the separate and combined effects of modern radiotherapy and cardiotoxic chemo- immuno-therapy on cardiac disease risk have only been addressed in a small number of studies generally with limited follow-up. Early detection of (sub)clinical cardiac damage may be important but currently there are no specific guidelines for screening on radiation-related cardiac diseases. At present, in order to prevent such diseases, screening can be aimed only at early detection and at treatment of general risk factors for CVD. Furthermore, there are indications of large inter-individual variation of susceptibility to treatment-related toxicity and of some variation of genetic susceptibility for radiation-related CVD, but further studies are needed [12].
Since clinical end-points often do not occur until at least 10–15 years after exposure and intervention before they occur may be useful, adequate imaging screening tools and surrogate markers are needed for treatment-related cardiac diseases.
Better knowledge concerning risk factors and mechanisms underlying radiation-related CVDs will contribute to primary and secondary prevention of long-term treatment complications in cancer survivors where exposure of the heart to radiation is unavoidable [6].
Cardiotoxicity following systemic treatment is typically associated with loss of myocardial mass, leading to progressive cardiac remodeling and dysfunction. Patients experiencing cardiotoxicity develop Heart Failure (HF) months to years after the initial cancer therapy and have a severely impaired cardiovascular prognosis [13]. Anthracyclines are a well-known example of cardiotoxicity; the pathophysiological mechanism is complex but involves dose-related myocardial cell death during cancer treatment and possibly an impairment of reparatory and homoeostatic mechanisms after the exposure to chemotherapy [14]. Signalling inhibitors -such as anti-HER2 compounds and angiogenesis inhibitors- were also found to induce cardiac dysfunction. However, in contrast to anthracyclines these drugs typically lead to cardiac dysfunction during cancer treatment with a high potential of reversibility [15]. Furthermore, recent data suggest that patients exposed to trastuzumab have a low risk of progressive cardiac disease even when followed for years after the initial cancer treatment. While cancer drug associated, irreversible cardiotoxicity has recently been termed Type I cardiotoxicity, the reversible form of cardiac dysfunction was named Type II dysfunction. Other cancer drug related cardiovascular side-effects with long-term implications for patients include BCR/ABL tyrosine kinase inhibitor-induced pulmonary hypertension and peripheral arterial occlusive disease [14,15]. These effects typically occur years into treatment with these compounds and the course of the disease remains unclear [11].
Androgen-Deprivation Therapy (ADT), which is a common treatment for prostate cancer, has been tentatively linked with an increased risk of cardiovascular disease.
Studies looking for links between CVD and ADT have reached conflicting conclusions. For instance, one meta-analysis found a 40 percent increased risk of non-fatal CVD in men with prostate cancer who had received ADT. On the other hand, an earlier study found no link at all between ADT and cardiovascular mortality.
It has therefore been difficult for researchers, so far, to draw accurate lines between heart health and ADT.
Studies have run into a range of problems: some primarily looked at older men, wherein heart conditions would already be more common, and some did not take information about the other medications that participants were taking.
And, even when links have been found, it is difficult to know whether ADT caused the CVD or simply worsened a pre-existing heart condition [16].
The study included 7,637 men who had recently been diagnosed with localized prostate cancer. Of these men, almost a third (30 percent) received ADT. They were followed-up for a maximum of 13 years.
After controlling for the variables described above — as well as others including body mass index (BMI) and smoking — individuals who had ADT and no pre-existing CVD had an 81 percent increased risk of heart failure [16].
Men who did have pre-existing CVD saw a 44 percent increase in their risk of arrhythmia. Similarly, the risk of conduction disorder (a problem with the way that electrical impulses move through the heart) tripled.
When discussing why the link between CVD and ADT might occur, the researchers cover a range of potential factors.
Firstly, testosterone deficiency increases fat mass, which is a risk factor for CVD. Also, men with low testosterone levels are more likely to have abnormal lipid profiles, increased levels of pro-inflammatory factors, and hypertension.
The authors hope that these results might help to identify prostate cancer patients who are more at risk of CVD. They can then give them regular cardiac check-ups, encourage them to exercise, and monitor their blood pressure and diabetes more closely [16].
Extensive knowledge has already been gained from the past concerning cancer treatment related cardiotoxicity, but there is much more that can be done, although medical practice continues to evolve and so the resulting data need to be interpreted with care. Better knowledge is needed of the late effects of modern systemic treatments and of radiotherapy on critical structures of the heart and of possible interactions between treatment modalities. This knowledge will contribute to a longer life expectancy and better quality of life of cancer survivors, with less treatment-related morbidity from other diseases [6].
References
- Early Breast Cancer Trialists Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and on 15-year survival: An overview of the randomised trials. Lancet 2005; 366: 2087-2106.
- Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994; 12: 447-453.
- Cardio-Oncology in the Netherlands.
- Kaitlynn Ely. Patients With Breast Cancer or Lymphoma Have Increased Risk of Heart Failure. 2018.
- Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ET. Cardiovascular complications of cancer therapy: Best practices in diagnosis, prevention, and management: Part 1. Journal of the American College of Cardiology. 2017; 70: 2536-2551.
- Aleman BM, Moser EC, Nuver J, Suter TM, Maraldo MV, et al. Cardiovascular disease after cancer therapy. European Journal of Cancer Supplements. 2014; 12: 18-28.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England Journal of Medicine. 2013; 368: 987-998.
- Bhatia N, Santos M, Jones LW, Beckman JA, Penson DF, et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. 2016; 133: 537-541.
- Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: A population-based study. BMC cancer. 2007; 7: 9.
- Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. Journal of Clinical Oncology. 1994; 12: 447-453.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, et al, Jensen MB. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England Journal of Medicine. 2013; 368: 987-98.
- Hilbers FS, Boekel NB, van den Broek AJ, van Hien R, Cornelissen S, et al. Genetic variants in TGFβ-1 and PAI-1 as possible risk factors for cardiovascular disease after radiotherapy for breast cancer. Radiotherapy and Oncology. 2012; 102: 115-21.
- Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC cancer. 2010; 10: 1-4.
- Eschenhagen T, Force T, Ewer MS, De Keulenaer GW, Suter TM, et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. European journal of heart failure. 2011; 13: 1-10.
- de Azambuja E, Bedard PL, Suter T, Piccart-Gebhart M. Cardiac toxicity with anti-HER-2 therapies-what have we learned so far?. Targeted oncology. 2009; 4: 77-88.
- https://www.medicalnewstoday.com/articles/319777.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.