Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case report
- |
- Open Access
- |
- ISSN: 2639-4383
Progressive Unmasking of the ARVC Phenotype Triggered by a Pulmonary Embolism
- Anura Malaweera*;
- Department of Cardiology, King’s College Hospital, London, UK.
- Daniel Sado;
- Department of Cardiology, King’s College Hospital, London, UK.
- Rachel Bastiaenen;
- Department of Cardiology, St Thomas’ Hospital, London, UK.
- Paul Scott
- Department of Cardiology, King’s College Hospital, London, UK.

| Received | : | Aug 04, 2021 |
| Accepted | : | Aug 26, 2021 |
| Published Online | : | Aug 30, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Malaweera A, Sado D, Bastiaenen R, Scott P. Progressive Unmasking of the ARVC Phenotype Triggered by a Pulmonary Embolism. Ann Cardiol Vasc Med. 2021: 4(1); 1052.
Keywords: ARVC; Pulmonary emboli; RV dysfunction; Ventricular tachycardia; Catheter ablation.
Abstract
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) is characterised by Right Ventricular (RV) dysfunction, ventricular arrhythmias, and usually presents before the age of 40. We report a case of the ARVC phenotype being unmasked by Pulmonary Emboli (PE) in late adulthood, manifesting as recurrent Ventricular Tachycardia (VT), which was successfully treated with catheter ablation.
Case report
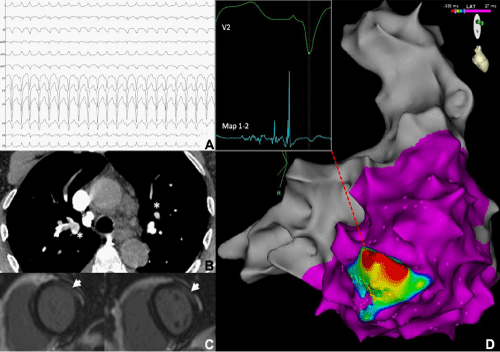
A 70-year-old endurance athlete with no significant past medical history presented with a 24-hour history of breathlessness. Clinical examination revealed he was tachycardic, hypoxic and hypotensive. A 12-lead ECG demonstrated a broad complex tachycardia at 162bpm with a superior axis and QRS transition at V5, consistent with VT with an origin in the right ventricle (Figure 1A). An emergency electrical cardioversion restored sinus rhythm and a baseline ECG revealed T-Wave Inversion (TWI) in the anterior chest leads. Bedside echocardiography showed RV pressure overload and a CTPA (Figure 1B) confirmed extensive bilateral Pulmonary Emboli (PE). He underwent thrombolysis, making a good recovery. He was initiated on Warfarin. A whole-body CT scan did not show any evidence of malignancy and no trigger for the PE was found.
A subsequent inpatient Cardiac MRI (CMR) revealed preserved biventricular function and no evidence of pulmonary hypertension or RV strain. There was transmural late enhancement of the Left Ventricular (LV) anterolateral wall with elevated native myocardial T2, suggesting myocardial oedema. However, coronary angiography demonstrated unobstructed coronaries and contrast bubble echocardiography demonstrated no intracardiac shunt. No significant change was seen on a CMR at 6-weeks.
18 months later he had another presentation with haemodynamically compromising VT, with an identical QRS morphology to the initial arrhythmia. This required further electrical cardioversion. Investigations, which included a CTPA, CT PET, V/Q scan, vasculitic screen and serum ACE, were negative. On repeat CMR (Figure-1C) RV systolic function was now impaired (ejection fraction of 39%), and the RV cavity was dilated (end diastolic volume of 103ml/m2) with basal hypokinesis. The LV late enhancement persisted in an epicardial distribution.
Further episodes of drug-refractory VT with the same QRS morphology occurred whilst an in- patient and therefore an urgent electrophysiological study with electroanatomical mapping (CARTO®, Biosense Webster) was performed. VT was easily induced and the earliest activation and fractionated electrograms were found to be at the endocardial aspect of the inferobasal RV free wall near the tricuspid annulus (Figure-1D). Ablation here terminated the tachycardia and a scar map revealed a large area of low voltage in the corresponding area of RV. A subsequent programmed ventricular stimulation was negative.
Genetic testing for Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) was negative, however the ARVC task force criteria (Major Criteria: VT arising from basal RV free wall, TWI in precordial leads on resting ECG; minor criteria: RV dilatation with basal hypokinesis and signal-averaged ECG demonstrating late potentials) were met [1]. An implantable cardioverter defibrillator was placed prior to discharge. No further VT has been detected after 3 years of follow-up.
Although it is well recognised that exercise may increase arrhythmic risk and structural changes in ARVC patients, the unmasking of ARVC by PE has not been reported. Our case underlines the complex interplay between right ventricular strain and the genesis of arrhythmias in ARVC.
Figure 1: 12-lead ECG at presentation (A). CTPA at presentation with extensive bilateral PE (B- asterisks). CMR at 18 months demonstrating RV dilatation with epicardial late enhancement, (C-arrowhead). Activation map shows the area of earliest activation with the corresponding electrogram from ablation catheter at RV basal inferior wall (D).
References
- Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/Dysplasia: Proposed modification of the task force criteria. Circulation. 2010.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.