Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Potentiating of Aging-Related Left Ventricular Hypertrophy to Secondhand Smoke Exposure Animal Model
- Jia-Ping Wu
- Research Center for Healthcare Industry Innovation, National Taipei University of Nursing and Health Sciences. No. 365, Mingde Rd, Beitou Dist, Taipei City 11219, Taiwan, R.O.C.

| Received | : | Dec 30, 2020 |
| Accepted | : | Feb 05, 2021 |
| Published Online | : | Feb 09, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Jia-Ping W. Potentiating of Aging-Related Left Ventricular Hypertrophy to Secondhand Smoke Exposure Animal Model. Ann Cardiol Vasc Med. 2021: 4(1); 1042.
Keywords: Secondhand smoke exposure; The left ventricle; Cardiac impaired; Left ventricular hypertrophy.
Abstract
Secondhand smoke (SHS) exposure is associated with an increased risk of coronary artery disease. The aim was to investigate the relationship of SHS exposure in aged mice left ventricle impaired. To explore cardiac remodeling of SHS exposure whether was exacerbated cardiac impaired, especially in the aged mice. The C57BL/6J mice were placed in a transparent exposure chamber, connected to the smoking device, and exposed to 15 cigarettes for 30 min/twice a day/week, for 1 month. Histopathologic of left ventricular sections were stained with hematoxylin-eosin staining (H&E) and Masson’s trichrome. Left Ventricular (LV) morphological variables assessed using H&E stained and mass weight changes. The left ventricular hypertrophy structures were measured by echocardiographic analysis. Results showed in male C57BL/6J mice group and aged mice in Secondhand Smoke (SHS) exposure group were observed LV wall and mass increased, collagen accumulatio, and extracellular space increased. From echocardiographic results, we found LV functions were apparently decreased, LV interventricular septum at systolic and diastolic diameters increased in the aged SHS group. We suggest that exposure to secondhand smoke increases the risk of heart diseases and coronary disease.
Introduction
Secondhand Smoke (SHS) exposure is associated with elevated risks of coronary heart disease and stroke. The risks associated with exposure were very similar to risks from light active smoking, despite the much greater exposure to tobacco smoke inactive smoking. However, acute exposure has been shown to increase platelet activity [1]. Experimental data also suggest that SHS exposure elevates aging-related left ventricular hypertrophy risk remains uncertain. Secondhand smoke exposure has been linked to harmful health outcomes which is an important cause of short-lifespan morbidity and mortality [2]. Aging is a physiological process involving progressive impairment of heart functions, due to an increasing vulnerability, which reduces the ability to survive [3]. However, it is not a clear pathological condition in aging exposure to SHS. The aim of this study was to examine SHS exposure in aging-related left ventricular hypertrophy of hearts. Secondhand smoke (SHS) exposure is the risk of coronary heart disease. SHS exposure is the combination of smoke given off by the burred end of a tobacco or cigarette product to exposure to the environment and the smoke exhaled by the smoker [4]. Slowly progressive age-related diseases are also chronic diseases [5]. A mature organism occurs normally the gradual changes in the structure over time and increases the probability of death. This growing process is unavoidable. These physiologic changes of old cardiac include left ventricular hypertrophy. Cardiovascular disease is a major risk factor for the aging cause of death. Aging changes in the elderly heart are associated with physiological left ventricular hypertrophy (LVH). However, SHS exposure is associated with pathological LVH [6]. Heart failure is a related change in cardiac morphology, including decreased in myocyte number, increased in myocyte size, decreased in matrix connective tissue, increased in left ventricular wall thickness [7,8-9]. SHS exposure may stimuli first induce a phase of cardiac hypertrophy, especially in left ventricles [10]. Aging changes may produce clinical heart disease and may mimic heart disease [11]. Therefore, we detected the molecular mechanisms behind the aging in SHS exposure treatment to identify pathological cardiac disease disorder and elusive.
Methods
Animals
Male C57BL/6J mice were purchased at 6 weeks of age from National Science Council Animal Center and used according to the guidelines of the Helsinki Declaration, and then male rats fed to 18 months of age at the animal center. Only male mice are used due to reducing the potential for variability resulting from gender-related differences in cardiac aging. Use the institutional animal care and committee (IACUC), Taiwan committee approved animal care and experiments. C57BL/6J mice (n=5) were housed in one cage in an environmentally controlled animal room. One group of mice of 6-weeks-old rats as our young, another group of mice of 18-months-old as our older age groups. Animal room temperature is maintained at 25°C, and relative humidity was approximately 40%.
Secondhand smoke (SHS) exposure
The old rats were placed in a whole-body transparent exposure chamber with a volume of approximately 95x85x85 cm, connected to a smoking device. Filtered air is introduced into the chamber at a low rate. Puffs of cigarette smoke were collected in the smoking chamber, being then thrown into the chamber for 30 minutes. The smoke is released at a rate of 15 cigarettes, twice a day in the morning and twice in the afternoon with 30 minutes rest intervals, until the end of 4 weeks.
Echocardiography
Animals used anesthetized with ketamine hydrochloride (50mg/kg) and xylazine hydrochloride (1mg/kg). Echocardiograms are performed on young adult and old mice at 4 weeks after secondhand smoke exposure (SHS) using a Hewlett-Packard Sonos 5500 ultrasound machine equipped with a 15-MHz transducer. M-mode image was recorded and analyzed offline. The following parameters were measured and calculated: left ventricular interior diastolic diameter (LVIDd), left ventricular interior systolic diameter (LVIDs), left ventricular interventricular septum at systolic and diastolic (LVIDs, LVIDd), left ventricular posterior wall thickness at systolic and diastolic (LVPWs, LVPWd), fractional shortening (FS%) and ejection fractional (EF%).
H&E and Masson’s Trichrome (MT) staining
The left ventricle cross-sections were sectioned into 10 μm thick and placed on slides, after perfusion fixation with buffered Formalin for 15 min. Slides deparaffinization and dehydration were performed. hematoxylin & eosin and Masson’s Trichome stained were prepared, incubated at room temperature. Color images of cross-sections were made at 400x total magnification using a Nikon E600. The left ventricular sections were processed for quantification of fibrosis. Masson’s trichrome stained were prepared. The volume of collagen of the cross-sectional area was measured and assessed by statistical analysis.
Statistically analysis
All data examined were expressed as mean ± SEM. Statistical analysis of the data was performed using Sigma Stat software. Comparison between group was made using one-way ANOVA test utilized with turkeys post hoc test. A p value of less than 0.05 and 0.01 was statistically significant.
Results
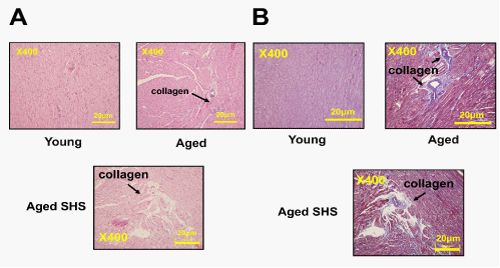
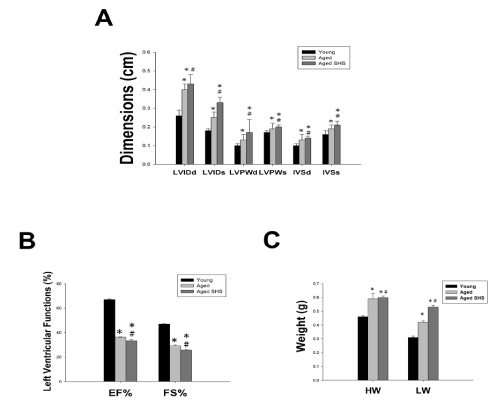
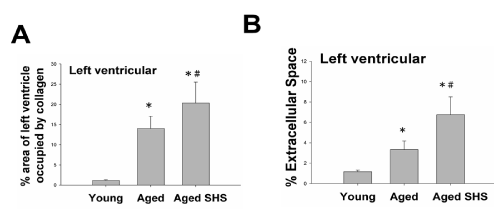
Histopathologic of a left ventricular cross-sectional analysis assessed cardiac changes in aged mice in secondhand smoke exposure by H&E stained and Masson’s stained. To investigate the effects of secondhand smoke (SHS) exposure on cardiac functions and structural changes were determined in mice model recommended for gerontological. Left ventricular cross-sections were stained with Masson’s trichrome or hematoxylin/eosin staining for visualization of morphology and identification of the location. A cross-sectional analysis assessed left ventricular changes in C57BL/6J mice and aged mice in the SHS exposure group. As shown in Figure 1, in aged mice and aged mice SHS exposure group, we also could observe ECM degradation resulted in collagen release in cardiomyocytes interstitial from H&E stained (Figure 1A) and collagen accumulation induced fibrosis (Figure 1B). Indeed, from Masson’s trichrome stained results, we could observe blue color staining in cross-sections in young, aged mice and aged SHS mice. Thus, left ventricular function and structures development of left ventricle on echocardiographic analysis in young, aged mice and aged SHS mice. The echocardiographic analysis is a primary imaging method in the assessment of cardiac impairment and function declined (Figure 2A). Parasternal long-axis and short-axis echocardiographic views in young, aged mice and aged SHS mice showing severe left ventricular hypertrophy. We found left ventricular wall thickness increased. However, from M-mode short-axis echocardiograms result taken proximal to the left ventricular deterioration in young, aged mice and aged SHS mice. Quantification of hypertrophy for young, aged mice and aged SHS mice are displayed in Figure 2A. Echocardiography of interventricular septal in systolic (IVSs), interventricular septal in diastolic (IVSd), left ventricular internal dimension at end systolic (LVIDs), left ventricular internal dimension at end diastolic (LVIDd), left ventricular posterior wall thickness in systolic (LVPWs) and left ventricular posterior wall thickness in diastolic (LVPWd) were presented in young, aged mice and aged SHS mice. The morphological variables obtained from the echocardiographic study are shown in Figure 2A. The aged mice SHS exposure had greater IVSs, IVSd, LVIDs, LVIDd, LVPWs and LVPWd dimensions compared with young mice. After exposure to SHS, the aged mice had statistically greater dimensions than nonsmoking aged mice did. This variable change was used to confirm the efficacy of the exposure of aged mice to SHS. In addition, considering the left ventricular variables, the ejection and shortening fractions were significantly declined. As Figure 2B shown, shortening (FS%) and ejection fraction (EF%) displayed a progressive impairment in aged mice and aged mice SHS exposure group. Figure 2C. Presents heart and left ventricular (LV) characteristics in young, aged mice and aged SHS mice. The whole heart weights of aged mice and aged mice in the SHS exposure were heavier than young mice. Aging and SHS exposure mice were also enhanced left ventricular weights (Figure 2C). However, bodyweight is easily caused by the gradual increase in the consequence of aging. To detect whether aged mice exposure to SHS led to left ventricular fibrosis exacerbated, we independently calculated the percentage of per cross-sectional area from H&E sections. Quantification of the percentage of the area of left ventricle occupied by collagen (%), collagen area was measured in aged mice and aged SHS mice (Figure 3A). The percentage of tissue attributed to collagen distribution increased more rapidly in the aged SHS group than in aged mice or young control mice (p<0.05). Furthermore, to detect whether SHS exposure led to left ventricular hypertrophy exacerbated, quantification of left ventricular muscle interstitial width of extracellular space, we found left ventricular muscle interstitial become broad resulted in the percentage of extracellular space (%) increased (p<0.05) (Figure 4B). We suggest SHS exposure led to left ventricular hypertrophy character change in the aging process.
Figure 1: Representative histopathological analysis of left ventricular cross-sections with hematoxylin & eosin (H&E) and Masson’s trichrome staining in young, aged mice and aged SHS mice. (A). Representative collagen accumulation in the left ventricle by hematoxylin & eosin staining of left ventricular sections in young, aged mice and aged SHS mice. Scale bars 20μm. The images of left ventricular architectures were magnified 400x. (B). Representative fibrosis in the left ventricle by Masson’s trichrome staining in young, aged mice and aged SHS mice. Scale bars 20μm. The images of left ventricular architectures were magnified 400x.
Figure 2: Left ventricular hypertrophy takes place in young, aged mice and aged SHS mice. (A). Representative M-mode echocardiograms taken proximal from young, aged mice and aged SHS mice. These images were obtained from short- axis imaging at the midpapillary level. Parasternal short-axis echocardiography views (up-panel), parasternal short-axis echocardiography views (down-panel). Interventricular septal in systolic (IVSs), interventricular septal in diastolic (IVSd), left ventricular internal dimension at end systolic (LVIDs), left ventricular internal dimension at end diastolic (LVIDd), left ventricular posterior wall thickness in systolic (LVPWs) and left ventricular posterior wall thickness in diastolic (LVPWd) shown in right panel. (B). Quantification of interventricular septal at diastolic and systolic, left ventricular internal dimension at end diastolic and systolic, left ventricular posterior wall thickness at diastolic and systolic, and the percentage of fractional shorting and ejection fraction. *p<0.05 compared with young mice. *p<0.05 compared with aged mice. (C). Quantification of heart weight and left ventricle weight statistical analysis. *p<0.05 compared with young mice. *p<0.05 compared with aged mice.
Figure 3: Histopathologic of left ventricular fibrosis and left ventricular hypertrophy in young, aged mice and aged SHS mice. (A). Quantification of percent of area of left ventricle occupied by collage. *p<0.05 compared with young mice. *p<0.05 compared with aged mice.(B). Quantification of percent (%) extramyocyte connective tissue space (area). *p<0.05 compared with young mice. *p<0.05 compared with aged mice.
Discussion
Secondhand smoke (SHS) has been linked to harmful health outcomes important cause of morbidity and mortality. However, there is no evidence that indicated that SHS exposure presents a challenging health hazard. In this study, we investigated the effects of SHS exposure associated with the elderly age, specifically in the left ventricles of male rats. In contrast, aging is a physiological process due to increasing injuries and vulnerability, which reduces the ability of organisms to survive [12]. The overall effect is highly debatable aging and disease. As age increases, whether there will occur diseases itself. The main characteristics associated with aging is a progressive decrease in physiological capacities [13,14]. As aging heart, demonstrated severe left ventricular chamber dilation, wall thinning and fibrosis, leading to congestive heart failure. We found that changes associated with SHS exposure led to cardiovascular pathological outcomes resulted in age-related disease exacerbated (Figure 1). These results demonstrated left ventricular hypertrophy in aged mice and aged mice exposure to SHS. From echocardiography results to determine left ventricular dimension, posterior wall thickness, interventricular septal at end-systole and end-diastole were increased, and left ventricular function declined (Figure 2). Stiffening of these fibers cause left ventricular fibrosis and could also affect the efficient functioning (Figure 3). SHS exposure is linked to a key risk factor for pathological hypertrophy associated with various cardiovascular disease risk factors. As the heart reaches senescence, it undergoes a modest degree of Heart failure [15,16]. It is now determined the differences in several signaling molecules play a unique role in the regulation of aged mice in the SHS exposure group. A good night’s sleep can help your body’s health. Sleep well can stimulate your brain to release hormones to encourage tissue growth [17,18]. With aging, try to avoid activities or beverages more before bed. Allowing your body to rest can help you to reduce blood pressure to speed your recovery time. A good night’s sleep is just as good effects as regular exercise and a healthy diet [19]. Good sleep has immediate anti-aging effects on your hormones, exercise performance and cardiovascular function [20]. With aging, good sleep quantity and quality, exercise better can help you be healthier and longevity [21]. If you want to optimize your health to lose weight, then getting a good night’s sleep is one of the most important things you can do. Regular training improves the vasodilatory properties of the vasculature thereby optimizing O2 transport throughout the body [22]. Exercise training is a physiological left ventricular hypertrophy adaptive response of the cardiomyocytes to physiological stresses which in response to increased workload [23,24]. Exercise training considers overlap exists between the mechanisms that control the pathological growth of the heart and the physiological growth of the heart [25]. Increased cardiomyocyte cell size and protein synthesis are properties of both physiological and pathological Left ventricular hypertrophy. Since left ventricular hypertrophy is a positive adaptation to exercise training, it appeared plausible that treatment during exercise could block the physiologic growth of the myocardium and be deleterious.
References
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, et al. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J Am Med Dir Assoc 2013; 13: S1525-8610.
- Wu JP, Che TT. Secondhand Smoke Exposure in Aging-related Cardiac Disease. Aging Dis 2013; 4: 127-133.
- Jiang D, Zheng D, Wang L, Huang Y, Liu H, et al. Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PLoS One 2013; 8: e59752.
- Flouris AD, Vardavas CI, Metsios GS, Tsatsakis AM, Koutedakis Y. Biological evidence for the acute health effects of secondhand smoke exposure. Am J Physiol Lung Cell Mol Physiol 2010; 298: L3-L12.
- Halaszynski T. Influences of the aging process on acute perioperative pain management in elderly and cognitively impaired patients. Ochsner J 2013; 13: 228-247.
- Bonomini F, Rodella LF, Moghadasian M, Lonati C, Rezzani R. Apolipoprotein E deficiency and a mouse model of accelerated liver aging. Biogerontology 2013; 14: 209-229.
- Wu JP, Hsieh CH, Ho TJ, Kuo WW, Yeh YL, et al. Secondhand smoke exposure toxicity accelerates age-related cardiac disease in old hamsters. BMC Cardiovasc Disord 2014; 14: 195.
- Zhu J, Rebecchi MJ, Wang Q, Glass PS, Brink PR, et al. Chronic Tempol treatment restores pharmacological preconditioning in the senescent rat heart. Am J Physiol Heart Circ Physiol 2013; 304: H649-H659.
- Wu JP, Hsieh DJ, Kuo WW, Han CK, Pai P, et al. Secondhand Smoke Exposure Reduced the Compensatory Effects of IGF-I Growth Signaling in the Aging Rat Hearts. Int J Med Sci 2015; 12: 708-718.
- Zhu J, Rebecchi MJ, Glass PS, Brink PR, Liu L. Cardioprotection of the aged rat heart by GSK-3beta inhibitor is attenuated: age-related changes in mitochondrial permeability transition pore modulation. Am J Physiol Heart Circ Physiol 2011; 300: H922-H930.
- Simkhovich BZ, Marjoram P, Poizat C, Kedes L, Kloner RA. Age-related changes of cardiac gene expression following myocardial ischemia/reperfusion. Arch Biochem Biophys 2003; 420: 268-278.
- Brink TC, Demetrius L, Lehrach H, Adjaye J. A e-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology 2009; 10: 549-564.
- Liu P, Xu B, Cavalieri TA, Hock CE. Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc Res 2002; 56: 443-453.
- Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, et al. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Arterioscler Thromb Vasc Biol 2012; 32: 1024-1031.
- Miyamoto A, Kawana S, Kimura H, Ohshika H. Impaired expression of Gs alpha protein mRNA in rat ventricular myocardium with aging. Eur J Pharmacol 1994; 266: 147-154.
- Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 2012; 16: 1492-1526.
- Corbi G, Conti V, Scapagnini G, Filippelli A, Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci 2012; 4: 768-778.
- Bard RL, Dvonch JT, Kaciroti N, Lustig SA, Brook RD. Is acute high-dose secondhand smoke exposure always harmful to microvascular function in healthy adults? Prev Cardiol 2010; 13: 175-179.
- Pacheco SA, Torres VM, Louro H, Gomes F, Lopes C, et al. Effects of occupational exposure to tobacco smoke: is there a link between environmental exposure and disease? J Toxicol Environ Health A 2013; 76: 311-327.
- Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes Rev 2012; 13: 58-68.
- Gielen S, Sandri M, Kozarez I, Kratzsch J, Teupser D, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation 2012; 125: 2716-2727.
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 2012; 67: 599-610.
- Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis 2011; 2: 158-173.
- Sheydina A, Riordon DR, Boheler KR. Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond) 2011; 121: 315-329.
- Faught BE, Flouris AD, Cairney J. Epidemiological evidence associating secondhand smoke exposure with cardiovascular disease. Inflamm Allergy Drug Targets 2009; 8: 321-327.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.