Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case Report
- |
- Open Access
- |
- ISSN: 2639-4383
Helping to Control Pulmonary Hypertension in a Trisomy 21 Infant with a Fenestrated Atrial Septal Defect Device
- Stephanie Saey;
- Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City IA, USA.
- Kurt Bjorkman;
- University of Iowa Stead Family Children’s Hospital, Department of Pediatrics, Division of Pediatric Cardiology, Iowa City, IA, USA.
- Jennifer R Maldonado;
- University of Iowa Stead Family Children’s Hospital, Department of Pediatrics, Division of Pediatric Cardiology, Iowa City, IA, USA.
- Daniel McLennan*
- University of Iowa Stead Family Children’s Hospital, Department of Pediatrics, Division of Pediatric Cardiology, Iowa City, IA, USA.

| Received | : | Dec 11, 2021 |
| Accepted | : | Jan 19, 2022 |
| Published Online | : | Jan 24, 2022 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Saey S, Bjorkman K, Maldonado JR, McLennan D. Helping to Control Pulmonary Hypertension in a Trisomy 21 Infant with a Fenestrated Atrial Septal Defect Device. Ann Cardiol Vasc Med. 2022; 5(1): 1057.
Keywords: Heart defects; Congenital; Cardiac catheter; Down Syndrome.
Abstract
As an alternative to surgical intervention, transcatheter closure of an atrial septal defect has become the standard treatment for patients with such defects. These procedures typically result in full closure of the defect with limited risks. However, in patients with ASD and coexisting pulmonary hypertension, it is necessary to keep a residual shunt as a decompression mechanism to maintain cardiac output in the setting of acute increases in pulmonary vascular resistance. We report the use of an Occlutech® fenestrated ASD device in a patient less than 1 year of age who had an ostium secundum ASD, Trisomy 21, and pulmonary hypertension. Use of the fenestrated device reduced the pulmonary over-circulation and associated symptoms while allowing for possible right-to-left shunting during times of high pulmonary arterial pressure.
Introduction
Transcatheter closure of an Atrial Septal Defect (ASD) is commonly carried out to reduce the progression of right sided heart dilation and failure, arrhythmias and Pulmonary arterial Hypertension (PH) secondary to excessive pulmonary flow [1-3]. However, in patients with ASD and coexisting conditions such as left ventricular dysfunction, right ventricular dysfunction, or pulmonary hypertension, complete occlusion of the atrial septum and removal of its ability to serve as a “pop-off” could have potentially fatal consequences [3,4] The process of creating handmade fenestrations in a commercially available ASD occluder device has been well described, but control over the size of the fenestration is limited and the patency is often difficult to maintain despite anticoagulation [5-7]. The Occlutech® Fenestrated Atrial Septal Defect (FASD) occluder (Occlutech® International, Helsingborg, Sweden) provides a manufactured alternative with demonstrated safety and clinical benefit in adults and older pediatric patients [4-8]. Here we report our experience using this device in an infant with Trisomy 21, an ASD, pulmonary hypertension, and left atrial hypertension secondary to left ventricular diastolic dysfunction to prevent further worsening of pulmonary hypertension while also allowing for atrial decompression in the setting of dynamic changes to Pulmonary Vascular Resistance (PVR).
Clinical summary
An 8kg 8-month-old female with Trisomy 21, ostium secundum ASD was evaluated by cardiology after spending several months in the hospital with respiratory failure and recurrent respiratory infections. On evaluation, she was noted to have PH and left atrial hypertension secondary to left ventricular diastolic dysfunction. She was started on diuretics, but had right ventricular dilation, biventricular hypertrophy, and abnormal ventricular septal wall motion. Thyroid levels were all within normal range (Thyroid Stimulating Hormone, Thyroxin 4). Diagnostic cardiac catheterization was performed and confirmed PH, elevated right heart pressures (mean of 11 mmHg) with mean pulmonary artery pressure of 27 mmHg, pulmonary wedge pressure of 12 mmHg, mean left atrial pressure of 12 mmHg, and PVR 3.91 indexed Woods units. Cardiac index based on Fick was 3.91 L/min/m2 with significant left-to-right shunt (Qp:Qs of 1.7:1). Due to the severity of respiratory process and cardiac findings of right heart dilation, right ventricular hypertrophy, left atrial hypertension secondary to mitral regurgitation and left ventricular diastolic dysfunction, partial closure of the defect with a FASD was decided. It is known that large shunting lesions, like this patient, contribute to worsening PH and lung disease. Local IRB and FDA approvals for compassionate use were acquired for use of the Occlutech®FASD occluder (Figure 1).
At 11 months old and 9.1 kg, the patient returned to the cath lab for FASD device closure. Pre-deployment Transesophageal Echocardiogram (TEE) confirm the ASD size (5.7-6.9 mm) (Figure 2). The total septal length was 25.9 mm with adequate rims-superior 5.9 mm, inferior 12.0 mm, aortic 3.8 mm, SVC 7.4 mm and IVC 7.4 mm (Figure 3). A 10mm Occlutech®FASD occluder with a 3mm fenestration was chosen and inserted following standard ASD closure guidelines [1]. Once the device was adequately placed, TEE confirmed adequate positioning with no interference of adjacent structures prior to release. The device conformed nicely to the anatomy of the atrial septum and was released without difficulty. Repeat echocardiography indicated good positioning and no residual shunt around the device with an expected shunt through the fenestration (Figure 4). Reducing the ASD defect by 75% of its original size. The procedure was well-tolerated. Chest X-ray and echocardiogram prior to discharge showed the device to be in good position with open atrial fenestration. The patient was discharged home one day after the procedure on aspirin and diuretics.
At 1-month follow-up, echocardiogram revealed normalization of the right heart size, with normal cardiac function. The device was well seated with a patent fenestration and left to right shunt. The patient was clinically improved and was continued on ASA and diuretics.
At 7 month follow up (18 months old) after the procedure, echocardiogram showed no atrial level shunt, normal right atrium and right ventricle size and function. The patient was off all medication and clinically much improved.
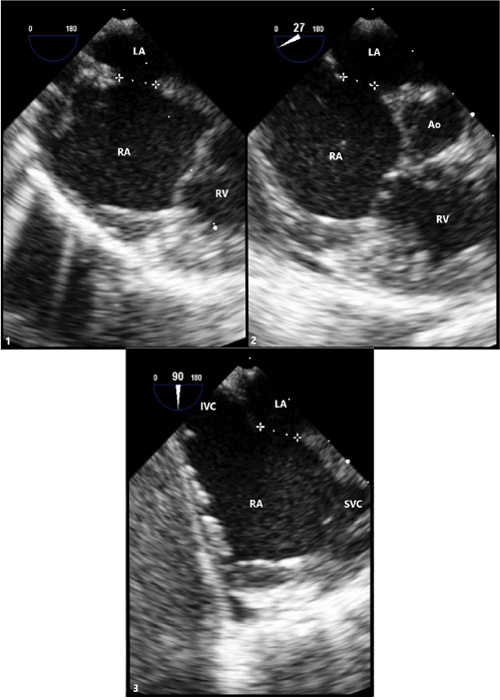
Figure 2: Transesophageal (TEE) Imaging of Secundum ASD
(1) TEE at 0° with defect measuring 6.7mm.
(2) TEE at 27° with defect measuring 5.7mm.
(3) TEE at 90° with defect measuring 6.9mm.
LA: Left Atrium; RA: Right Atrium; RV: Right Ventricle; Ao: Aortic Root; IVC: Inferior Vena Cava; SVC: Superior Vena Cava.
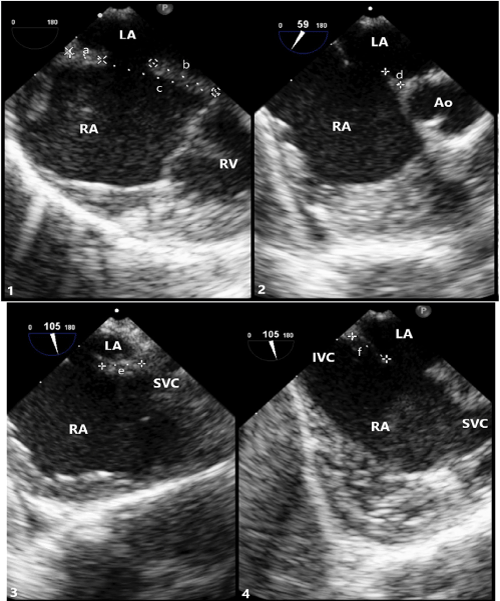
Figure 3: Transesophageal (TEE) Imaging of Secundum ASD Rims
(1) TEE at 0° showing superior rim measuring 5.9mm(a), inferior rim measuring 12.0mm(b), and total septal length measuring 25.9mm(c).
(2) TEE at 59° showing aortic rim measuring 3.8mm(d).
(3) TEE at 105° showing SVC rim measuring 7.4mm(e). (4) TEE at 105° showing IVC rim measuring 7.4mm(f).
LA: Left Atrium; RA: Right Atrium; RV: Right Ventricle; Ao: Aortic Root; IVC: Inferior Vena Cava; SVC: Superior Vena Cava.
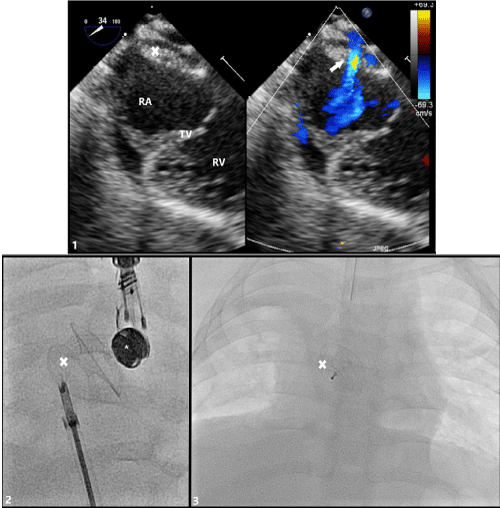
Figure 4: Transesophageal (TEE) Imaging of Secundum ASD with Occlutech® Fenestrated ASD Closure Device in Place
(1) TEE probe at 34° with color compare showing device in appropriate position fully covering secundum ASD with residual left-to-right shunt through fenestration (arrow). (2) Angiogram demonstrating appropriate placement of fenestrated ASD closure device just prior to release from delivery sheath and cable. (3) Post-procedure posterior-to-anterior chest x-ray image of appropriate device placement
RA: Right Atrium; TV: Tricuspid Valve; RV: Right Ventricle; X: Occlutech® Fenestrated ASD Closure Device; *: TEE Probe.
Discussion
This case highlights the novel use of an Occlutech® fenestrated ASD occluder for treatment of significant trans-septal shunt when a residual pop off is still required as was the case for infant with both left-ventricular dysfunction and concern for pulmonary arterial hypertension related to trisomy 21. The device minimizes the degree of atrial shunting and pulmonary over-circulation but maintains the possibility of a pop-off with dynamic PVR.
Fenestrated atrial septal devices were designed to allow continued shunts in patients with either left or right atrial hypertension [8]. In our patient the combination of a large atrial septal defect and left atrial hypertension led to significant pulmonary over-circulation and worsening pulmonary hypertension. Therefore, the fenestration worked as both a pop off for the LA hypertension or pulmonary hypertension during potential pulmonary hypertension crisis. Once implanted, reduction in atrial level shunting significantly reduced symptoms without the patient being compromised.
The Occlutech® fenestrated ASD occluder is an ASD device with a built-in fenestration allowing for controlled reduction in flow. Prior to development of this device, fenestrations needed to be created by either placing a coronary stent though the device over a wire once the device was in place or by cauterizing a hole through the device before placement [9]. Devices with set fenestrations remove the risks associated with creating fenestrations during the intervention, such as excess stent material in the left atrium. Additionally, the device design minimizes the chance of complete closure of atrial communication through endothelialization over the fenestration by promoting tissue growth away from the fenestration. It allows for a set size fenestration, which can be catered for different patients, depending on how much flow is required between the atria [10].
A recent paper by Vettukattil et al. demonstrated the use of a fenestrated ASD device in treating patients with PH and a large AS [8]. In that paper the smallest patient who had the device implanted was 5 years old. Here we described how it can be implanted in patients less than 1 year old and less than 10 kg safely and effectively. The device design permits delivery through moderate sized sheath (9Fr). The flexible delivery cable allows for tension free placement when deployed across the atrial septum. This allows for better stability and echo assessment. When released there is minimal change in conformation. This means there is negligible stress placed on the heart during delivery.
It should be noted that the patient’s fenestration did close spontaneously after 7 months; however, this is a longer period of time than that previously reported with handmade fenestrations [5,8,9].
Conclusion
Occlutech fenestrated ASD device is a very useful and safe device. It allows for reduction in atrial level shunting in a minimally invasive controlled manner. This paper has shown that it can be used in symptomatic patients as small as 9kg and possibly even smaller.
Acknowledgements
TEE images were obtained by Dr. Umang Gupta.
References
- Jung SY, Choi JY. Transcatheter closure of atrial septal defect: principles and available devices. Journal of thoracic disease. 2018; 10: S2909-S2922.
- Fraisse A, Latchman M, Sharma SR, Bayburt S, Amedro P, et al. Atrial septal defect closure: indications and contra-indications. Journal of thoracic disease 2018; 10: S2874-S2881.
- Jain S, Dalvi B. Atrial septal defect with pulmonary hypertension: When/how, can we consider closure? Journal of thoracic disease. 2018; 10: S2890-S2898.
- Gonzalez-Barlatay F, Fournier A, Raboisson MJ, Dahdah N. Atrial Septal Defect closure with Occlutech® ASD fenestrated device in a child with severe pulmonary hypertension. Pediatr Cardiol. 2017; 38: 202-205.
- Kretschmar O, Sglimbea A, Corti R, Knirsch W. Shunt reduction with a fenestrated Amplatzer device. Catheter Cardiovasc Interv. 2010; 76: 564-571.
- Kenny D, Cao Q, Hijazi M. Fenestration of Gore Helex Septal Occluder device in a patient with diastolic dysfunction of left ventricle. Catheter Cardiovasc Interv. 2011; 78: 597-598.
- Abdelkarim A, Levi D, Tran B, Ghobrial J, Aboulhosn J. Fenestrated transcatheter ASD closure in adults with diastolic dysfunction and/or pulmonary hypertension: case series and review of the literature. Congenit Heart Dis. 2016; 11: 663-671.
- Vishal Kaley, Nagib Dahdah, Amal El-Sisi, Jochen Grohmann, Eric Rosenthal, et al. Atrial septal defect-associated pulmonary hypertension: Outcomes of closure with a fenestrated device. Advances in Pulmonary Hypertension. 2019; 18: 4-9.
- Skinner GJ, Tulloh RM, Tometzki AJ, Shulze-Neick I, Morgan GJ. Atrial septal defect closure with an Amplatzer septal occlude fenestrated with a coronary stent in a child with pulmonary arterial hypertension. Cardiol Young. 2013; 23: 692-696.
- McLennan D, Ivy D, Gareth J. Transvenous implantation of the Occlutech Atrial Flow Regulator: preliminary results from swine models. Congenital Heart Disease. 2019; 14: 819-831.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.