Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Evaluation of Serum Osteopontin Levels as Biomarkers for Children with Kawasaki Disease
- Andre Jakob*;
- Department of Pediatric Cardiology and Pediatric Intensive Care, Ludwig-Maximilians-University of Munich, Germany.
- Eva Schachinger;
- Department of Congenital Heart Disease and Pediatric Cardiology, University Heart Heart Centre Freiburg-Bad Krozingen, University of Freiburg, Germany.
- Simon Klau;
- Institute for Medical Information Processing, Biometry and Epidemiology, Ludwig-Maximilians-University of Munich, Germany.
- Markus Hufnagel;
- Department of Pediatrics and Adolescent Medicine, Division of Pediatric Infectious Diseases and Rheumatology, Germany.
- Natascha van der Werf Grohmann;
- Department of Pediatrics and Adolescent Medicine, University Medical Center, University of Freiburg, Germany.
- Brigitte Stiller;
- Department of Congenital Heart Disease and Pediatric Cardiology, University Heart Heart Centre Freiburg-Bad Krozingen, University of Freiburg, Germany.
- Anja Tengler;
- Department of Pediatric Cardiology and Pediatric Intensive Care, Ludwig-Maximilians-University of Munich, Germany.
- Sarah Ulrich
- Department of Pediatric Cardiology and Pediatric Intensive Care, Ludwig-Maximilians-University of Munich, Germany.

| Received | : | Nov 20, 2020 |
| Accepted | : | Dec 15, 2020 |
| Published Online | : | Dec 18, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Jakob A, Schachinger E, Klau S, Hufnagel M, Natascha van der WG, et al. Evaluation of Serum Osteopontin Levels as Biomarkers for Children with Kawasaki Disease. Ann Cardiol Vasc Med. 2020: 3(1); 1035.
Keywords: Osteopontin; Kawasaki disease; Biomarkers
Abstract
Objectives: A reliable laboratory test for verifying diagnosis of Kawasaki Disease (KD) has yet to be developed. Given recent increases in KD-like disease among pediatric COVID-19 patients, such a test is of particular interest. Here, we investigate the bone-associated protein osteopontin (OPN)-a protein involved in vascular injury and remodeling-as a potential biomarker for KD, a vascular inflammatory disease.
Methods: We analyzed serum OPN levels of 62 KD patients and compared them to 63 Healthy (HC) and 11 Febrile Controls (FC). Osteopontin’s correlation with the time course of the disease, its association with clinical parameters such as coronary artery aneurysm, its refractivity to IVIG, and its connection to routine inflammatory parameters were assessed. Additionally, in combination with routine laboratory parameters, OPN properties for predicting KD were investigated.
Results: During the acute phase of KD, OPN levels were especially elevated as compared to the convalescent phase. In addition, OPN was positively correlated (r= 0.42) with CRP in KD patients. Among the three groups (KD, HC and FC), OPN levels were highest among KD patients. This differed significantly from the HC group. However, mean OPN did not considerably exceed FC mean levels. In analyzing osteopontin´s potential to predict KD, we observed that the Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curve was 0.73% for OPN alone, but 93% for OPN in combination with other laboratory values.
Conclusion: Our study indicates that osteopontin becomes elevated during the acute phase of KD. In combination with the evaluation of other routine laboratory values, looking at osteopontin levels may help to differentiate KD from other febrile diseases.
Introduction
A generalized vasculitis in children, Kawasaki Disease (KD) primarily affects the coronary arteries. In Germany, KD incidence is estimated to be 6.4-7.2/100.000 [1]. Although the disease’s molecular basis is not fully understood, evidence suggests development of the disease to be based upon an interplay between microbial infection and genetic predisposition. However, external factors such as breastfeeding and vitamin D appear to be protective [2]. Different signaling pathways that induce inflammation e.g., interleukin (IL) -1, IL-6, and Tumor Necrosis Factor α (TNFα) -are known to be involved in KD pathogenesis [3]. Immune activation during the acute phase of KD has been observed in small to medium-sized arteries [4] and is associated with endothelial cell damage. KD is diagnosed via clinical criteria, however, this can be unreliable. Unfortunately, clinical diagnosis is additionally problematic, because late recognition is associated with worse outcomes [3]. Research targeting identification of KD-specific biomarkers has the aim of facilitating earlier, more effective diagnosis and treatment. Especially high-risk KD patients, i.e., those not responsive to a first intravenous immunoglobulin therapy (IVIG), may benefit from additional, early medical interventions, because these patients have a higher chance of developing Coronary Artery Aneurysms (CAA) [5]. So far, however, reliably identifying high-risk KD patients only has been possible in Japanese children, and not in Caucasian ones [5]. As part of the search for potential biomarkers indicating KD activity, we undertook an evaluation of Osteopontin (OPN). OPN is a member of the small, integrin-binding, N-linked glycoprotein family of proteins. OPN interacts with various pro-inflammatory cytokines, including TNF-α and IL-1β, to increase OPN expression [6]. A strong chemoattractant for macrophages and T cells7, OPN plays a key role in the migration and vascular tissue accumulation of macrophages8. For these reasons, OPN may represent an interesting target protein for Kawasaki disease. Potentially, OPN could provide indication not only of systemic, but also of vascular wall inflammation.
After investigating serum levels of osteopontin in acute and convalescent KD patients, we compared the results to healthy (HC) and Febrile Controls (FC). Clinical and echocardiographic findings refractivity to IVIG and the presence of CAA, as well as routine laboratory values were investigated in relation to the osteopontin levels.
Material and methods
Patients
KD patients were recruited from a nationwide, prospective surveillance study through the hospital-based German Pediatric Surveillance Unit (ESPED) [1]. Pseudonymous identifiers were employed in the ESPED reports. A standardized questionnaire explicitly requesting information on all parameters needed for the purpose of validating KD diagnosis was sent to the reporting physicians. For numeric parameters such as laboratory values, the reporting physicians were asked to document the measured values along with their respective units. For categorical parameters, respondents were provided the option to check either “yes” or “no”. American Heart Association (AHA) guidelines [9] were used to classify complete versus incomplete KD cases. Diagnosis of CAA was determined based upon the echocardiographic judgment of the reporting physician. Refractory KD cases were defined as those with fever that persisted for over 36 hours following IVIG treatment. This group of patients was given a second IVIG course. As a part of this study, reporting physicians were also invited to send in blood serum for osteopontin analysis. Serum samples of 62 KD patients were available. Patient age ranged from two months to 15 years, with a mean age of 4.5 years. Of these patients, 40 (65%) were male. In addition, 63 Healthy Controls (HC) and 12 Febrile Controls (FC) were investigated. The healthy controls had a mean age of 7.1 years and 34 (54%) were male. Among the febrile controls, mean age was 4.2 years and six (50%) were male. HC subjects were defined as those without a history of oncologic, cardiac, autoimmune or rheumatic diseases. They were recruited from a pool of patients undergoing minor elective surgery and/or routine blood sampling. Additionally, it was required that they be free of febrile disease at the time of blood drawing. FC subjects were defined as previously healthy children who recently had experienced at least three days of fever and at least one clinical sign of KD (exanthema, conjunctival injection, erythematous/edematous hands of feet, enanthema or cervical lymphadenopathy). The FC subjects were recruited from the pediatric emergency department at the Freiburg University Hospital. Diagnosis of FC subjects included: proven viral infections in six cases (adenovirus n= 2, Influenza B n= 3, bocavirus n= 1); pyelonephritis (n= 1); mastoiditis (n= 1); appendicitis (n= 1); and fever of unknown origin (n= 3).
Laboratory methods
Serum from KD patients was prepared via centrifugation. The treating hospitals sent us the serum at ambient temperature via 24-hour delivery service. We then immediately stored it at -80oC until informed consent from the parents had been obtained and we could proceed with analysis. An enzyme-linked immunoassay kit (human osteopontin ELISA Kit, Sigma-Aldrich, Darmstadt, Germany) was used to determine osteopontin levels according to the producer’s standard protocol. Prior to use, the assay was validated by taking pooled serum from adult patients with coronary artery disease and examining intra- and inter-assay precision.
Statistical analysis
Statistical analysis was performed using the statistical software R (R-3.4.1). The impact of patient age and sex, as well as the day of illness when blood was collected, was assessed by linear regression analysis. Pearson and Spearman correlation coefficients were used to evaluate the correlation between osteopontin levels and standard inflammatory markers. Student t-test was used to test whether OPN levels differed between KD patients with versus without CAA. A p-value of <0.05 was considered to be significant. Tukey´s honest significant difference method was used to compare the mean values of KD, HC and FC patients. Additionally, we assessed osteopontin’s value in differentiating KD patients from FC patients by determining the Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curves, as well as by calculating sensitivity and specificity. Approval for the study was provided by the Ethics Committee of the University of Freiburg, in compliance with the ethical standards of the Helsinki Declaration.
Results
Following the American Heart Association criteria for KD [3], 62 children were diagnosed as having KD, including 14 (22%) classified as incomplete KD. Seventeen (27%) patients had CAA during the acute phase of the disease, 14/59 (24%) of the patients were refractory to IVIG, and three patients received no IVIG treatment, because KD diagnosis only was established after fever had resolved. For information on the characteristics of baseline patients, including clinical data and standard laboratory values, see (Table 1).
In both KD and HC groups, neither sex nor age appeared to exert significant influence on OPN levels (data not shown). However, when linear regression analysis was applied to the HC group, we observed a slight tendency (a factor of 0.054 with every patient year (p= 0.074)) towards decreasing OPN levels with (Figure 1).
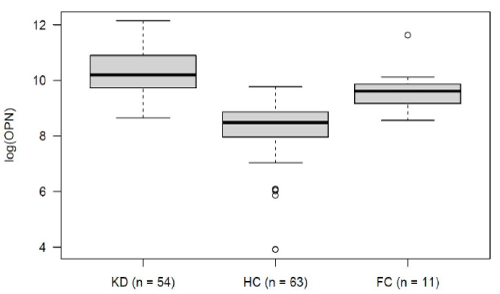
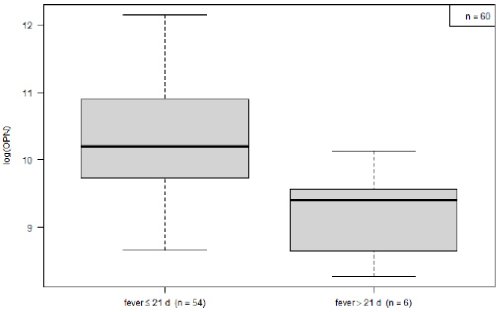
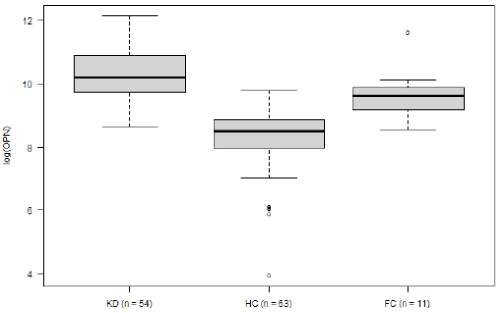
Within the KD group, OPN levels decreased over the course of the disease. Mean logarithmic OPN levels were significantly higher during the acute phase (n= 54) as compared to the convalescence phase (10.25 vs. 9.23, p= 0.03; (Figure 2). For KD’s acute phase, we analyzed OPN levels in relation to development of CAA and to IVIG-refractivity. Neither IVIG treatment nor the presence of CAA displayed a significant impact on OPN levels (data not shown). The association between OPN levels and routine laboratory values collected from: (1) all KD patients, (2) during the disease’s acute stage and (3) before IVIG treatment. These were then analyzed according to the Pearson and Spearman coefficient. Across all groups, OPN showed a moderately positive correlation with CRP, and a negative correlation with serum albumin levels (Table 2). When comparing logarithmic OPN levels among acute-stage KD patients, HC and FC, we found levels to be highest among KD patients (Figure 3). These levels differed significantly from HC (mean difference HC vs. KD -1.9, 95% CI: -2.4; -1.5; p<0.000001). However, FC patients also displayed significantly higher OPN levels than did HC (mean difference FC vs. HC 1.39; 95% CI 0.6; 2.1; p= 0.0001). Meanwhile, in this regard, KD and FC did not exhibit significant differences (FC vs. KD -0.57; 95%CI -1.36; 0.21; p= 0.2).
Logistic regression analysis displayed a correlation between increased risk of KD and elevated logarithmic OPN levels (OR 2.38; p= 0.058). According to the ROC curve (AUC 0.71), OPN alone was able to differentiate between KD and FC. This was with a sensitivity of 74% and specificity of 73%. When taking OPN into consideration in addition to the available FC laboratory values (CRP, leukocytes, hemoglobin and thrombocytes), the KD predictive quality increased AUC of ROC to 93%, sensitivity to 94% and specificity to 73%.
Figure 1: Osteopontin levels in healthy children (n= 63). There is a tendency towards decreasing OPN levels with a factor of 0,054 with every patient year (p= 0,074).
Figure 2: Comparison of osteopontin leves in acute (<22 d, n= 54) vs. convalescent phase of the disease (>= 22 d, n= 6).
Table 2: Correlation of osteopontin levels and routine laboratory values from all patients with KD, KD during the acute stage of disease and prior to IVIG treatment.
Discussion
In this explorative study, we analyzed osteopontin levels in order to examine their clinical relevance for KD. During the acute disease phase, osteopontin values were significantly higher than during the convalescent phase. KD´s OPN values during the acute phase also were significantly higher as compared to HC. However, when clinical relevance as a KD-specific biomarker was depreciated, these values did not substantially differ from FC. Furthermore, our investigation showed that OPN levels were neither associated with the presence of CAA, nor were they refractory to IVIG therapy, although both of these outcomes are of highly clinical importance to KD patients. Instead of being KD-specific, osteopontin seems to represent a general inflammatory marker. This hypothesis also is supported by a positive correlation to CRP.
Osteopontin is known to interact with various cytokines, such as TNFα and IL-1β, which are involved in the general pro-inflammatory state of KD [6,10]. In addition, OPN is related to atherosclerotic-specific inflammatory mediators, with one of the most essential being angiotensin II (Ang II). Through the Renin Angiotensin Aldosterone System (RAAS), Ang II significantly interacts with the cardiovascular system and thereby promotes the development of atherosclerosis [11]. Under atherosclerosis conditions, OPN expression in the arterial wall is considerably increased by Ang II [12]. Osteopontin itself acts as a strong chemoattractant for macrophages and T-lymphocytes and therefore appears to promote inflammatory infiltrates in vascular plaques. In addition, osteopontin seems to play a key role in vascular calcification [13]. Coronary artery calcification, especially that found in giant CAA, also has been found in KD patients [14,15]. In-vitro analysis was able to relate the vascular calcification in KD to OPN. KD plasma induced the expression of OPN in human coronary artery smooth muscle cells at a higher rate than did plasma of other febrile diseases [16]. In vivo studies, however, will be needed in order to further analyze this effect. In our study, we examined whether OPN was associated with the existence of CAA. We found no significant effect. However, our study population, in particular when considering those with CAA, was small in number. Therefore, we cannot exclude the potential of OPN-KD interaction. Future studies-ones that include serial measurements and long-term follow up in a larger study population-may be better suited to determine osteopontin’s role in the short- and long-term pathogenesis of CAA.
Although logarithmic OPN levels did not differ between KD and FC patients, higher OPN levels (OR 2.38, p= 0.058) correlated with an increased risk for KD. Furthermore, as the ROC curve revealed, it appears that OPN alone has discriminative properties, ones that were heightened when combining OPN with available routine laboratory values. Because KD diagnosis is easier to establish when complete clinical symptoms are present, analyzing only those KD patients who had incomplete KD could be clinically meaningful. However, due to our small patient cohort and the related need for our analysis to be interpreted with caution, further subdividing the KD group would not have been adequate for statistical purposes.
Numerous studies have analyzed the association of different parameters as KD-specific biomarkers. Acute inflammatory biomarkers such as erythrocyte sedimentation rate (ESR), CRP and white blood cell count have been found to be significantly more elevated in KD patients as compared to febrile, non-KD controls [17,18]. However, because common inflammatory parameters are elevated during acute inflammatory diseases, they cannot be used to differentiate between KD and other febrile diseases, most particularly those with high-level inflammation. With the exception of the ESR value, which is unreliable following the administration of IVIG [18], common inflammatory parameters may serve as valuable parameters during clinical disease management. Another promising target includes cardiac markers. Acute myocarditis is known to accompany KD during the acute phase of the disease. In addition, NT-proBNP, creatinine kinase-MB and serum cardiac troponin I (cTnI) all have been involved in KD [19]. In terms of identifying KD, the most promising candidate seems to be NT-ProBNP. Although metaanalysis [20] supports this hypothesis, using NT-ProBNP to monitor disease activity has limitations. In addition to the above, proteomic studies also have identified biomarkers for diagnosing KD. However, to date none of these biomarkers have been validated for clinical use [21].
Strengths and limitation
This explorative study evaluates the impact of KD on osteopontin by comparing KD values to values from healthy children, as well as to those from febrile children with other diseases. To our knowledge, ours is the first analysis of OPN as a potential biomarker not just for KD cases in particular, but for children in general. Comparative normal values among children do not exist. Blood samples were generated from a prospective nationwide surveillance study in Germany. Although the exact timepoint of blood sample collection was documented, unfortunately there was no standardized protocol. As a result, the timepoints at which the blood samples were drawn were heterogeneous, both during the acute disease phase and during the convalescence phase. In addition, it was not possible to obtain a serial measurement in order to elucidate osteopontin’s association with the clinical course of KD. The number of patients our study included, especially those with CAA or who were refractory to IVIG, was small. As a result, our study likely is underpowered for the purpose of drawing definitive conclusions about OPN’s value. For the patients in the FC group, only blood cell counts and CRP were available. Unfortunately, other laboratory values were not available to help us further distinguish between KD and FC.
Conclusion
Our results showed elevated OPN levels in KD. Although absolute OPN levels were highest in KD, they did not significantly differ from FC patients. Nevertheless, higher OPN levels were associated with an increased risk for KD and therefore may be helpful in the discrimination of KD. Additional analyses with larger study cohorts are necessary to further investigate osteopontin’s role as a potential KD-specific biomarker.
References
- Jakob A, Whelan J, Kordecki M, Berner R, Stiller B, et al. Kawasaki Disease in Germany: A Prospective, Population-based Study Adjusted for Underreporting. Pediatr Infect Dis J. 2016; 35: 129-134.
- Meyer K, Volkmann A, Hufnagel M, Schachinger E, Klau S, et al. Breastfeeding and vitamin D supplementation reduce the risk of Kawasaki disease in a German population-based case-control study. BMC Pediatr. 2019; 19: 66.
- McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017; 135: e927-e999.
- Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, et al. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989; 2: 1298-1302.
- Jakob A, von Kries R, Horstmann J, Hufnagel M, Stiller B, et al. Failure to Predict High-risk Kawasaki Disease Patients in a Population-based Study Cohort in Germany. Pediatr Infect Dis J. 2018; 37: 850-855.
- Kaomongkolgit R, Manokawinchoke J, Sanchavanakit N, Pavasant P, Sumrejkanchanakij P. Fibronectin supports TNF-alpha-induced osteopontin expression through beta1 integrin and ERK in HN-22 cells. Arch Oral Biol. 2010; 55: 101-107.
- Wolak T, Sion-Vardi N, Novack V, Greenberg G, Szendro G, et al. N-terminal rather than full-length osteopontin or its C-terminal fragment is associated with carotid-plaque inflammation in hypertensive patients. Am J Hypertens. 2013; 26: 326-333.
- Lund SA, Wilson CL, Raines EW, Tang J, Giachelli CM, et al. Osteopontin mediates macrophage chemotaxis via alpha4 and alpha9 integrins and survival via the alpha4 integrin. J Cell Biochem. 2013; 114: 1194-1202.
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004; 114: 1708-1733.
- Salvi V, Scutera S, Rossi S, Zucca M, Alessandria M, et al. Dual regulation of osteopontin production by TLR stimulation in dendritic cells. J Leukoc Biol. 2013; 94: 147-158.
- Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002; 89: 3A-9A.
- deBlois D, Lombardi DM, Su EJ, Clowes AW, Schwartz SM, et al. Angiotensin II induction of osteopontin expression and DNA replication in rat arteries. Hypertension. 1996; 28: 1055-1063.
- Shao JS, Sierra OL, Cohen R, Mecham RP, Kovacs A, et al. Vascular calcification and aortic fibrosis: a bifunctional role for osteopontin in diabetic arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011; 31: 1821-1833.
- Kahn AM, Budoff MJ, Daniels LB, Jimenez-Fernandez S, Cox AS, et al. Calcium scoring in patients with a history of Kawasaki disease. JACC Cardiovasc Imaging. 2012; 5: 264-272.
- Ino T, Shimazaki S, Akimoto K, Park I, Nishimoto K, et al. Coronary artery calcification in Kawasaki disease. Pediatr Radiol. 1990; 20: 520-523.
- Chang SF, Liu SF, Chen CN, Kuo HC. Serum IP-10 and IL-17 from Kawasaki disease patients induce calcification-related genes and proteins in human coronary artery smooth muscle cells in vitro. Cell Biosci. 2020; 10: 36.
- Huang MY, Gupta-Malhotra M, Huang JJ, Syu FK, Huang TY. Acute-phase reactants and a supplemental diagnostic aid for Kawasaki disease. Pediatr Cardiol. 2010; 31: 1209-1213.
- Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, et al. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011; 30: 1022-1026.
- Kim M, Kim K. Elevation of cardiac troponin I in the acute stage of Kawasaki disease. Pediatr Cardiol. 1999; 20: 184-188.
- Lin KH, Chang SS, Yu CW, Lin SC, Liu SCet alC. Usefulness of natriuretic peptide for the diagnosis of Kawasaki disease: a systematic review and meta-analysis. BMJ Open. 2015; 5: e006703.
- Kimura Y, Yanagimachi M, Ino Y, Aketagawa M, Matsuo M, et al. Identification of candidate diagnostic serum biomarkers for Kawasaki disease using proteomic analysis. Sci Rep. 2017; 7: 43732.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.