Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Research Article
- |
- Open Access
- |
- ISSN: 2639-4383
Evaluating the Role of Deferred Stenting in ST-Segment Elevation Myocardial Infarction Patients Presented with High Thrombus Burden: A Systematic Review and Meta-Analysis
- Xiangming Hu;
- The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China; Department of Cardiology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
- Xing Yang;
- Department of Cardiology, Guangdong Provincial People’s Hospital Zhuhai Hospital (Zhuhai Golden Bay Center Hospital), Zhuhai, China.
- Xida Li;
- Department of Cardiology, Guangdong Provincial People’s Hospital Zhuhai Hospital (Zhuhai Golden Bay Center Hospital), Zhuhai, China.
- Haojian Dong*;
- Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China.
- Yingling Zhou*
- Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China; Southern Medical University, Guangzhou, China.

| Received | : | Dec 09, 2020 |
| Accepted | : | Jan 22, 2021 |
| Published Online | : | Jan 27, 2021 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Hu X, Yang X, Li X, Dong H, Zhou Y. Evaluating the Role of Deferred Stenting in ST-Segment Elevation Myocardial Infarction Patients Presented with High Thrombus Burden: A Systematic Review and Meta-Analysis. Ann Cardiol Vasc Med. 2021: 4(1); 1039.
Abstract
Purpose: This meta-analysis was performed to assess the benefits of deferred stenting (DS) vs. Immediate Stenting (IS) in the ST-Segment Elevation Myocardial Infarction (STEMI) patients presented with high thrombus burden.
Methods: Cochrane Library, Embase, Pubmed, Web of Science and China National Knowledge Internet (CNKI) were searched for eligible literature.
Results: Seven Randomized Controlled Trials (RCTs) and 3 prospective cohort studies were included in this meta-analysis. Significant difference was observed in the incidence of no-/slow-reflow compared between DS and IS in RCTs (RR= 0.34, 95%CI [0.20, 0.59], P<0.001, I2= 0.0%) but not in prospective cohort studies. Meanwhile, the TIMI 3 flow (For RCTs: RR= 1.29, 95% CI [1.13, 1.48], P<0.001, I2= 0.0%; For prospective cohort studies: RR= 1.19, 95%CI [1.08, 1.32], P = 0.001, I2= 0.0%) and MBG (For RCTs: WMD= 0.70, 95% CI [0.57, 0.82], P< 0.001; For prospective cohort studies: WMD= 0.57, 95% CI [0.36, 0.79], P<0.001) after stent implantation were significant higher in patients treated with DS than those with IS. No significant differences were observed in major adverse cardiac events.
Conclusion: In patients with STEMI appeared angiographic high thrombus burden, the DS strategy may be considered a preferable option than IS. However, more evidence is necessary to evaluate long-term benefits.
Introduction
Primary Percutaneous Coronary Intervention (pPCI) is a standard treatment for patients with ST-Elevation Myocardial Infarction (STEMI). However, pPCI has yet a serious unsolved problem known as the no-/slow-reflow phenomenon, which occurs in 5-25 % of STEMI patients after Immediate Stenting (IS) implantation [1]. Acute reduction of blood flow in Infart-Related Artery (IRA) during pPCI results in “second attack” for the ischemic heart, bringing severe periprocedual complications including enlarging infarct size thus aggravating the decrease of cardiac function [2-3]. The cause of no-/slow-reflow might be platelet/endothelial activation, vasospasm, inflammatory response and myocardial edema explained by Abbate’s group via animal studies [4]. In clinical practice, High Thrombus Burden (HTB) was identified as an independent predictor of no-/slow-reflow [5-7]. Previous research studies have suggested that excessive interventions in HTB state, especially stent implantation, might inevitably produce small thrombus and release atherosclerotic substances [8], both of which were able to embolize the distal vessels, inducing inflammation, interstitial oedema, and vasospasm to damage microcirculation, and further, leading to no-/slow-reflow [9-11]. So far, attempts to avoid embolization by using mechanical and manual thrombectomy and distal protection haven’t shown desirable result [12-15].
Firstly reported by Isaaz, et al., [16], the concept of Minimalist Immediate Mechanical Intervention (MIMI) at early stage of STEMI ahead of stenting has attached much attention. MIMI provides good prerequisites for Deferred Stenting (DS) implantation. In recent years, there emerged an increasing number of clinical reports on the application of DS showing positive prognosis comparing to IS for STEMI patients with HTB [16-20]. After a continuous blood flow has been maintained in the IRA, DS could prevent embolisation, and thus potentially improve clinical outcome. This prevention might be caused by reduced microvascular obstruction and increased myocardial salvage. Several articles have analyzed the difference between DS and IS in patients with STEMI and HTB, in which there were various research designs, methods and conclusions. A meta-analysis is needed to analyze the special angiographic HTB group of patients who might benefit from DS. Hence, we systematically reviewed relevant studies comparing deferred and immediate stenting strategy in STEMI patients with HTB.
Methods
This article was conformed to PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement for reporting systematic reviews and meta-analyses of population-based studies [21].
Screening methods
We searched for population-based studies including Randomized Controlled Trial (RCT) and observational study that compared deferred and immediate stenting in STEMI patients with HTB. Cochrane Library, Embase, Pubmed, Web of Science and China National Knowledge Infrastructure (CNKI) were searched for relevant articles with the key words of “delayed”, “deferred”, “postponed”, “two stage”, “stent”, “percutaneous coronary intervention”, “PCI”, “STEMI”, “ST-segment Elevation Myocardial Infarction”, “thrombus burden”, “thrombus load”, from January 1, 2000 to October 1, 2020. During the search process, no restrictions concerning language or publication status were imposed.
Inclusion and exclusion criteria
The inclusion criteria are as follows: RCT or non-randomized, prospective, observational study design; Study compared deferred and immediate stenting in the patients with STEMI (symptom onset <12 hours) and high thrombus burden (Gibson thrombus score [22] ≥2 or high-burden thrombus formation [23]) at first angiography; MIMI strategy before deferred stenting was carried out: attained Thrombolysis In Myocardial Infarction (TIMI) 2 or 3 flow after Percutaneous Transluminal Coronary Angioplasty (PTCA), thrombus aspiration, or other methods without stenting; Study reported at least one of the following outcomes: no-/slow-reflow, TIMI flow and Myocardial Blush Grade (MBG) after the stent implantation, Major Adverse Cardiac Events (MACE) at the follow-up period. The exclusion criteria was that patients in the study were treated with intravenous thrombolysis or had a history of coagulation disorder. In terms of article type, case reports and case series were excluded for the representativeness of large populations is unknown.
Definition
No-flow was defined as TIMI= 0/1 flow after stenting implantation and slow-reflow was defined as TIMI = 2 flow after stenting implantation. MACE included events of cardiac death, non-fatal myocardial infraction and target vessel revascularization that reported by each research. Gibson thrombus score was defined as: 1) TIMI thrombus score 0, no cineangiographic characteristics of thrombus are present, 2) TIMI thrombus score 1, possible thrombus is present, with such angiography characteristics as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion suggestive but not diagnostic of thrombus, 3) TIMI thrombus score 2, there is definite thrombus, with greatest dimensions ≤1/2 the vessel diameter, 4) TIMI thrombus score 3, there is definite thrombus but with greatest linear dimension >1/2 but <2 vessel diameters, 5) TIMI thrombus score 4, there is definite thrombus, with the largest dimension ≥2 vessel diameters, 6) TIMI thrombus score 5, there is total occlusion [22]. High-burden thrombus formation including: 1) cutoff pattern of occlusion in the IRA, 2) accumulated thrombus (>5 mm) proximal to the occlusion, 3) presence of floating thrombus, 4) persistent dye stasis distal to the obstruction, 5) Reference Lumen Diameter (RLD) of the IRA ≥4 mm, 6) incomplete obstruction with presence of accumulated thrombus more than three times the RLD of the IRA [23].
Data extraction and quality assessment
The information of the study characteristics (e.g. author, year of publication), patient characteristics (e.g. age, gender), angiographic feature (e.g. no-/slow-reflow phenomenon, MBG) and clinical outcome (e.g. MACE) from each eligible studies were extracted. For data with multiple follow-up times, we chose the longest one. Two researchers independently screened the searched studies for study inclusion, a third researcher was involved when there was a discrepancy.
Quality control
The Cochrane Collaboration’s tool were used to quality assessment for RCTs, which includes the following items: allocation sequence generation, allocation concealment, participant masking, personnel and outcome assessors, completeness of outcome data, and selective outcome reporting [24]. Newcastle-Ottawa Scale (NOS) was selected for quality assessment of observational studies, which refers to selection, comparability and outcome [25].
Statistical analysis
Meta-analyses were performed for each of the outcomes stratified by study design (observational studies or RCTs). The random effects model was used for all analyses because it was the most applicable and conservative method for comparing heterogeneity between different reports. For continuous outcomes, we calculated Weighted Mean Differences (WMD) and 95% Confidence Intervals (CI). For dichotomous outcomes we calculated relative risks (RR) and 95% CIs using Mantel–Haenszel approach. Heterogeneity was assessed using the I2 statistic, with values <25%, 25% to 50%, >50% indicating low, moderate, and high heterogeneity, respectively. Publication bias were assessed using funnel plot and Egger’s test, and P<0.05 was considered the existence of significant publication bias. Duval and Tweedie’s Trim and Fill yields an unbiased estimate of effect sizes after adjusting for potential publication bias [26]. All statistical analyses were conducted using STATA 15 and Revman 5.3.
Results
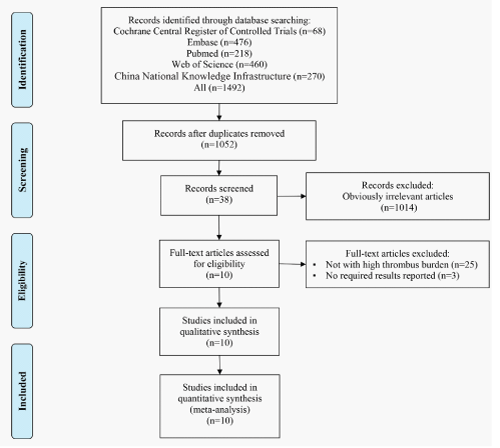
Search results and characteristics of included studies: The study inclusion process was illustrated in (Figure 1). We identified 7 randomized controlled trials involving 518 patients [27-33] and 3 prospective cohort studies involving 235 patients [19-20,34] in this systematic review. Baseline information of included studies were presented in Table 1 and Table 2. The delay time for DS ranged from 8 hours to 14 days and the follow-up period ranged from 14 days to 21 months. Most population were males and the average age varying from 54 to 69 years among studies. Of all patients, 30% to 64% had hypertension, 14% to 37% had diabetes mellitus, 20% to 77% had dislipidemia, 25% to 75% had smoking history, 5% to 20% had previous myocardial infraction, 4% to 72% had multi-vessel coronary disease.
Results of quality assessment
The quality assessment on the reviewed RCTs by Cochrane Collaboration’ s tool was shown in Supplementary (Figure 1). All of the trials showed performance bias and unclear risk of detection bias. One trial showed attrition bias. For prospective cohort studies, as shown in Table 3, we used Newcastle-Ottawa Scale (NOS) for quality assessment. All studies got the total scores of 9.
Efficiency of DS vs IS
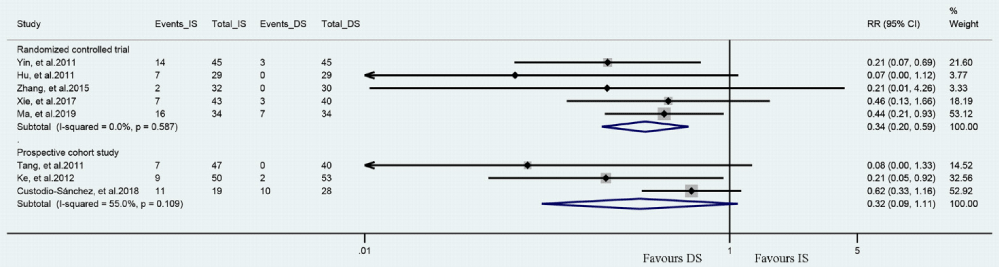
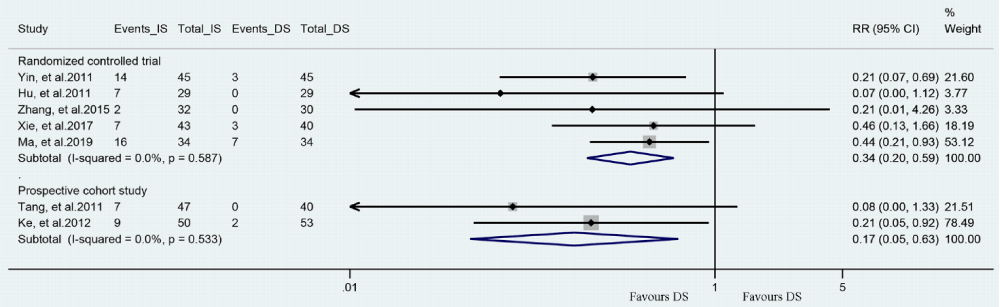
Meta-analysis about the no-/slow-reflow: There were 8 out of the 10 reviewed studies assessed incidence of no-/slow-reflow in patients had DS or IS (Figure 2). Meta-analysis of 5 RCTs shown DS group had a significantly lower incidence of no-/slow-reflow compared to IS group (RR = 0.34, 95%CI [0.20, 0.59], P < 0.001; P for Heterogeneity = 0.587, I2 = 0.0%). Meta-analysis of 3 prospective cohort studies demonstrated similar result but a wide CI was insignificant (RR = 0.32, 95%CI [0.09, 1.11], P = 0.072; P for Heterogeneity = 0.109, I2 = 55.0%).
Table 3: Quality assessment of prospective cohort studies (Newcastle-Ottawa Scale Criteria).
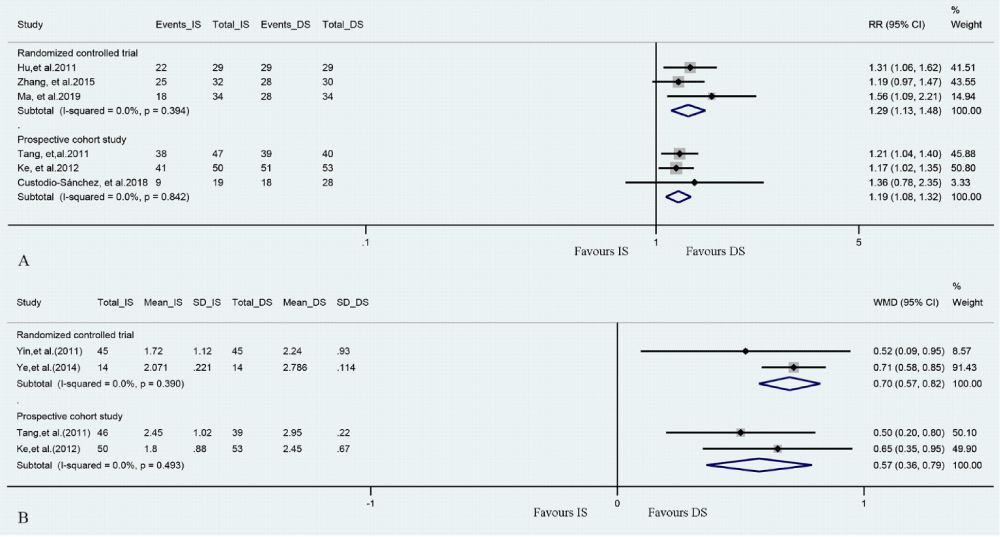
3.3.2 Meta-analysis about the TIMI 3 flow. Meta-analysis of 3 RCTs and 3 prospective cohort studies assessed instant TIMI 3 flow after DS or IS implantation (Figure 3A). DS group showed better TIMI 3 flow both in RCTs (RR= 1.29, 95%CI [1.13, 1.48], P<0.001, P for Heterogeneity= 0.394, I2= 0.0%) and prospective cohort studies (RR= 1.19, 95% CI [1.08, 1.32], P= 0.001, P for Heterogeneity= 0.842, I2= 0.0%).
Meta-analysis about the MBGMeta-analysis of 2 RCT and 2 prospective cohort studies assessed WMD of MBG in patients had DS or IS (Figure 3B). DS group was found to be significantly related to a superior level of MBG both in RCTs (WMD= 0.70, 95% CI [0.57, 0.82], P < 0.001, P for Heterogeneity= 0.390, I2= 0.0%) and in prospective cohort studies (WMD= 0.57, 95% CI [0.36, 0.79], P<0.001, P for Heterogeneity= 0.493, I2= 0.0%).
Meta-analysis about MACE
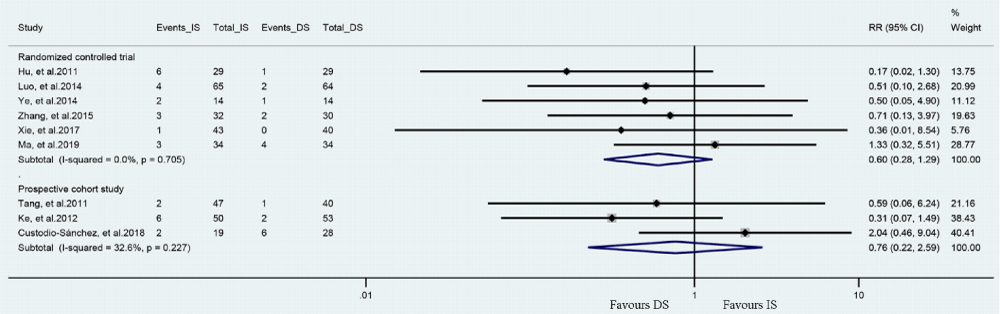
Six RCTs and 3 prospective cohort study assessed the incidence of MACE in patients had DS or IS (Figure 4). The meta-analysis showed that DS group tend to have a lower occurrence of MACE versus IS (For RCTs: RR= 0.60, 95%CI [0.28, 1.29], P= 0.190, P for Heterogeneity= 0.705, I2= 0.0%; for prospective cohort studies: RR= 0.76, 95% CI [0.22, 2.59], P= 0.665, P for Heterogeneity= 0.227, I2= 32.6%).
Results of sensitivity analysis and publication bias
Figure 3: (A) Forrest plot for TIMI 3 flow in DS vs. IS groups. (B) Forrest plot for MBG in DS vs. IS groups.
A sensitivity analysis for each meta-analysis with at least three studies was carried out by removing each of the study to assess for its influence on the overall effect value. The result demonstrated that I2 of sensitivity for no-/slow-reflow changed from 55.0 to 0.0% when removed the study of Custodio-Sanchez, et al., which indicated that the heterogeneity was mainly due to this study. The forest plot without Custodio-Sánchez, et al., was shown in Figure 5 and the result was similar. Funnel plots and Egger’s tests for each of the outcomes were performed to detect the publication bias (Supplementary Figure 2A-C). Visually, the result of no-/slow-reflow was asymmetry in the funnel plot, suggesting the possible risk of publication bias. In addition, the result of the Egger’s test also indicated the potential risk of publication bias (P=0.007). Trim-and-fill analysis procedure identified and filled four imputed studies to generate symmetrical funnel plot (Supplementary Figure 2D). Meta-analysis combing these four studies showed similar results (adjusted RR = 0.46, 95% CI: [0.30, 0.69], P<0.001; P for Heterogeneity= 0.362, Q=12.02). And each study had no significant effect on RR when removed individually.
Discussion
STEMI is a serious health issue known for its high mortality rate due to acute occlusion of coronary vessel. Even though the rapid recovery of antegrade flow by stenting, there is still a group of patients showed indirect signs of ischemia, such as changes in electrocardiogram and decline in cardiac function. Angiography imaging of these patients also showed no-/slow-reflow phenomenon with evidence of distal embolism, which denies the benefits of early reperfusion treatment and remain at higher risk for short and long term mortality [35].
In addition to shortening the time of reperfusion and careful mechanical intervention, the treatment of HTB at first angiography has become another difficult problem in preventing no-/slow-reflow. Even with the current use of drug-eluting stents, HTB is still an independent predictor of MACE in STEMI patients with a high incidence of no-/slow-reflow [36]. Since thrombectomy was no longer recommended as a routine therapeutic measure [12-14], adequate restoring of the blood flow and efficient anti-thrombotic therapy before implanting stenting play a pivotal role in reducing HTB state. And this is also reflected the concept of MIMI at early stage of STEMI. Thus, it seems that DS strategy can both reduce thrombus burden and increase myocardial salvage [37]. But until now, it is not clear whether DS or IS will give better prognosis to STEMI patients with HTB.
Our meta-analysis has shown that DS significantly attenuated the occurrence of no-/slow-reflow presented by the increase of TIMI 3 flow and MBG in STEMI patient with HTB. For short-term prognosis, DS was prone to decrease MACE. The reduction of no-/slow-reflow might be explained by the “transition period” that DS provided for the restoring of cardiovascular homeostasis, during which thrombus was sufficiently dissolved, hypercoagulability was down-regulated and inflammation was cooled down [38-40]. As for potential improvement of MACE, reduced thrombus burden and sufficient myocardial tissue perfusion prevent the recurrence of MI and target vessel revascularization. So the better cardiac outcome is easily to be understood because of more myocardial viability and less ventricular remodeling. Although DS showed advantage in these outcomes, its security is also what we need to concern. In the original researches, there was no recurrent ischemia event reported during the waiting period before DS [19-20,28-29]. We considered that this may benefit from proper use of antiplatelet drugs and adequate elimination of the thrombus burden, which also provided a safety guarantee for the choice of DS.
In the large four RCTs (DEFER-STEMI, DANAMI 3-DEFER, MIMI and INNOVATION study [41-44]) compared DS and IS strategy in STEMI patients, they couldn’t find benefits of DS compared with IS. None of these studies considered high thrombus burden as a condition for selecting patients, and therefore the potential benefits of DS could not be denied. A previous meta-analysis [45] focusing on these four RCTs used meta-regression analysis and found that compared with patients who had low thrombus burden at baseline, a favorable treatment effect on no-/slow-reflow with DS was seen in participants with a baseline HTB. The result indirectly reminded us that DS might be a feasible and effective strategy for patient with these characteristic. Years later, another meta-analysis [46] specially compared DS with IS in patients with HTB and the results were supportive of DS strategy in reducing no-/slow-reflow and MACE. Yet, the original research and population that analysis chose were not so appropriate, for example, patients were not STEMI or already underwent initial thrombolysis. Our study focused those homogeneous patients who have a clear diagnosis of STEMI with high thrombus burden at the first angiography and without intravenous thrombolysis, given that the use of thrombolytic drugs could influence the judgment of HTB. Besides, we conducted a publication bias analysis to ensure the results more objective. Our results suggested that the DS strategy for patients with HTB needs to be conducted more selectively. Although it significantly reduced the incidence of no-/slow-reflow and increased blood perfusion, different from previous analysis, its safety and incidence of MACE need to be further observed.
This meta-analysis has several limitations. Firstly, there was a publication bias in the result of no-/slow-reflow, but after trim-and-fill analysis procedure, the adjusted RR was similar with the unadjusted one. Therefore, these finding should be interpreted with cautions. Secondly, most of the included studies were from China, so the extrapolation value of conclusions is limited. Meanwhile, small population of patients enrolled might have induced possible imprecise estimates in terms of differences between DS and IS. Thirdly, the time period from the initial reperfusion to stenting in the reviewed studies were different (ranging from 8 hours to 14 days) and the follow-up times varied over these studies (ranging from 14 days to 1 year), which would have an influence on prognosis. We expect well-designed multicenter randomized controlled trials focusing on high thrombus burden populations could further establish the benefits for DS than IS in STEMI patients with HTB.
In conclusion, the present study showed that DS was more beneficial than IS in patients with STEMI and HTB, for reduction of no-/slow-reflow phenomenon, increase of TIMI 3 flow and MBG after stenting. Potential benefits of MACE and safety evaluation need further study to clarify.
Funding
Our research was supported by The National Key Research and Development Program of China (No. 2016YFC1301202).
References
- Ito H, Okamura A, Iwakura K, Masuyama T, Hori M, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996; 93: 1993-1999.
- Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004; 109: 1121-1126.
- Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998; 97: 765-772.
- Abbate A, Kontos MC, Biondi-Zoccai GG. No-reflow: the next challenge in treatment of ST-elevation acute myocardial infarction. Eur Heart J. 2008; 29: 1795-1797.
- Kirma C, Izgi A, Dundar C, Tanalp AC, Oduncu V, et al. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J. 2008; 72: 716-721.
- Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, et al. Angiographic Morphologic Features of Infarct-Related Arteries and Timely Reperfusion in Acute Myocardial Infarction. Chest. 2002; 122: 1322-1332.
- Napodano M, Ramondo A, Tarantini G, Peluso D, Compagno S, et al. Predictors and time-related impact of distal embolization during primary angioplasty. Eur Heart J. 2009; 30: 305-313.
- Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc Interv. 2017; 10: 215-223.
- Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol. 2012; 52: 8738-8782.
- Reffelmann T, Kloner RA. The “no-reflow” phenomenon: basic science and clinical correlates. Heart. 2002; 87: 162-168.
- Lim SY. No-reflow phenomenon by intracoronary thrombus in acute myocardial infarction. Chonnam Med J. 2016; 52: 38-44.
- Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, et al. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. 2006; 114: 40-47.
- Kelbaek H, Terkelsen CJ, Helqvist S, Lassen JF, Clemmensen P, et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention. J Am Coll Cardiol. 2008; 51: 899-905.
- Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008; 371: 1915-1920.
- Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction. The INFUSE-AMI randomized trial. JAMA. 2012; 307: 1817-1826.
- Isaaz K, Robin C, Cerisier A, Lamaud M, Richard L, et al. A new approach of primary angioplasty for ST-elevation acute myocardial infarction based on minimalist immediate mechanical intervention. Coron Artery Dis. 2006; 17: 261-269.
- Cafri C, Svirsky R, Zelingher J, Slutky O, Kobal S, et al. Improved procedural results in coronary thrombosis are obtained with delayed percutaneous coronary interventions. J Invasive Cardiol. 2004; 16: 69-71.
- Meneveau N, Seronde MF, Descotes-Genon V, Dutheil J, Chopard R, et al. Immediate versus delayed angioplasty in infarct-related arteries with TIMI III flow and ST segment recovery: a matched comparison in acute myocardial infarction. Clin Res Cardiol. 2009; 98: 257-264.
- Tang L, Zhou SH, Hu XQ. Fang ZF, Shen XQ. Effect of delayed vs immediate stent implantation on myocardial perfusion and cardiac function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention with thrombus aspiration. Can J Cardiol. 2011; 27: 541-547.
- Ke D, Zhong W, Fan L, Chen L. Delayed versus immediate stenting for the treatment of ST-elevation acute myocardial infarction with a high thrombus burden. Coron Artery Dis. 2012; 23: 497-506.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62: 1006-1012.
- Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001; 103: 2550-2554.
- Yip HK, Chen MC, Chang HW, Hang CL, Hsieh YK, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002; 122: 1322-1332.
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56: 455-463.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. 2018.
- Hu LQ, Gu Y, Zeng K, Gao B. Effect of delayed percutaneous coronary angioplasty with delayed stent implantation on the prognosis of patients with acute ST-segment elevation myocardial infarction. Shanghai Med J. 2011; 34: 54-57.
- Yin D, Zhu H, Zhou XC, Jia YQ, Cui HS, et al. Delayed stent implantation following primary percutaneous coronary intervention for ST elevation myocardial infarction in patients with severe massive thrombus. Heart. 2011; 97: A147.
- Luo XL, Liu Q, Wang LL, Weng JX, Zuo HH, et al. Effect of delayed stent placement on the prognosis of acute myocardial infarction with high thrombus burden and ST-segment elevation. Chin J Intervent Cardiol. 2014; 22: 697-701.
- Zhang P, Xie Q, Cheng Y, Zhuo YF, Huang BS, et al. Thrombus aspiration and tirofiban pretreatment for postoperative delayed stent implantation in the treatment of 30 patients with acute myocardial infarction. Herald of Medicine. 2015; 34: 1606-1609.
- Ma DM, Ye SQ, Fan ZC. Application of delayed stent in acute myocardial infarction with high thrombus burden. Chongqing Medicine. 2019; 48: 503-505.
- Xie HX, Deng SH, Huang L, Cheng ZQ, Han R, et al. Application of delayed stent placement in patients with acute thrombosis and acute ST-segment elevation myocardial infarction. The Journal of practical medicine. 2017; 33: 1791-1794.
- Ye JC, Chen S. Application of delayed stent implantation in emergency percutaneous coronary intervention for patients with acute thrombosis and acute ST-segment elevation myocardial infarction. Fujian Med J. 2014; 36: 36-38.
- Custodio-Sanchez P, Damas-De Los Santos F, Pena-Duque MA, Coutino-Castelan D, Arias-Sánchez E, et al. Deferred versus immediate stenting in patients with ST - segment elevation myocardial infarction and residual large thrombus burden reclassified in the culprit Lesion. Arch Cardiol Mex. 2018; 88: 432-440.
- Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis. 2015; 108: 661-674.
- Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, et al. Angiographic Stent Thrombosis After Routine Use of Drug-Eluting Stents in ST-Segment Elevation Myocardial Infarction.J Am Coll Cardiol. 2007; 50: 573-583.
- Kelbak H, Engstrom T, Ahtarovski KA, Lonborg J, Vejlstrup N, et al. Deferred stent implantation in patients with ST-segment elevation myocardial infarction: a pilot study. EuroIntervention. 2013; 8: 1126-1133.
- Piorkowski M, Fischer S, Stellbaum C, Jaster M, Martus P, et al. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets? J Am Coll Cardiol. 2007; 49: 1035-1042.
- Kunichika H, Ben-Yehuda O, Lafitte S, Kunichika N, Peters B, et al. Effects of glycoprotein IIb/IIIa inhibition on microvascular flow after coronary reperfusion.A quantitative myocardial contrast echocardiography study. J Am Coll Cardiol. 2004; 43: 276-283.
- Kloner RA, Dai W. Glycoprotein IIb/IIIa inhibitors and no-reflow. J Am Coll Cardiol. 2004; 43: 284-286.
- Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, et al. A Randomized Trial of Deferred Stenting versus Immediate Stenting to Prevent No or Slow-Reflow in Acute ST-Segment Elevation Myocardial Infarction (DEFER-STEMI). J Am Coll Cardiol. 2014; 63: 2088-2098.
- Kelbak H, Hofsten DE, Kober L, Helqvist S, Klovgaard L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet. 2016; 387: 2199-2206.
- Belle L, Motreff P, Mangin L, Range G, Marcaggi X, et al. Comparison of Immediate With Delayed Stenting Using the Minimalist Immediate Mechanical Intervention Approach in Acute ST-Segment–Elevation Myocardial Infarction The MIMI Study. Circ Cardiovasc Interv. 2016; 9: e003388.
- Kim JS, Lee HJ, Woong Yu C, Kim YM, Hong SJ, et al. INNOVATION Study (Impact of Immediate Stent Implantation Versus Deferred Stent Implantation on Infarct Size and Microvascular Perfusion in Patients With ST Segment–Elevation Myocardial Infarction). Circ Cardiovasc Interv. 2016; 9.
- Cassese S, Belle L, Ndrepepa G, Bosson JL, Fusaro M, et al. Deferred versus immediate stenting in primary PCI: A collaborative meta-analysis of randomized trials with cardiac magnetic resonance imaging data. Can J Cardiol. 2018; 34: 1573-1580.
- Sun B, Liu J, Yin H, Yang S, Liu Z, et al. Delayed vs. immediate stenting in STEMI with a high thrombus burden. Herz. 2019; 44: 726-734.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.