Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Case Report
- |
- Open Access
- |
- ISSN: 2639-4383
Effects of SGLT2 Inhibitor on Neurohormonal Activity and Electrolyte Management in an Elderly Patient with Type 2 Diabetes Mellitus and Acute Heart Failure: A Case Report
- Hajime Kataoka
- Department of Internal Medicine, Nishida Hospital, Tsuruoka-Nishi-Machi 2-266, Saiki-City, Oita 876-0047, Japan.

| Received | : | Nov 28, 2020 |
| Accepted | : | Dec 26, 2020 |
| Published Online | : | Dec 30, 2020 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Kataoka H. Effects of SGLT2 Inhibitor on Neurohormonal Activity and Electrolyte Management in an Elderly Patient with Type 2 Diabetes Mellitus and Acute Heart Failure: A Case Report. Ann Cardiol Vasc Med. 2020: 3(1); 1038.
Abstract
Introduction: Effects of a sodium-glucose cotransporter-2 inhibitor (SGLT2i) on changes in the neurohormonal activity were investigated in an 85-year-old patient with Type 2 Diabetes Mellitus (T2DM) and acute Heart Failure (HF).
Case presentation: While maintaining the background medications constant, we started her on the SGLT2i empagliflozin 10 mg/d to treat the worsening HF and diabetic derangement. One week later, her body weight was markedly reduced by 3.6 kg, and HF-related physical signs were ameliorated, but serum b-type natriuretic peptide concentration was only slightly reduced. Despite attaining marked diuresis, the changes in the hemoglobin and hematocrit values from baseline were mild, indicating less vascular contraction after SGLT2i administration. Serum sodium and chloride concentrations were increased on day 7 and further increased on day 28 under SGLT2i treatment. Treatment induced a marked reduction in the plasma renin activity on day 7, which was further reduced to a near normal level on day 28.
Conclusion: According to the “chloride theory” for HF pathophysiology, reduced activation of the renin-angiotensin-aldosterone system by an SGLT2i could be expected by (1) preserving or enhancing chloride ions supplied to the macula densa and (2) retaining the vascular volume as a result of the enhanced serum chloride concentration.
Introduction
Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) is an anti-hyperglycemic drug with accompanying diuretic action [1,2]. Its pharmacologic properties of diuretic action are not yet fully clarified. Recent clinical study [3] reported that an SGLT2i diuretic enhances or regains serum chloride concentrations in patients with Type 2 Diabetes Mellitus (T2DM)/Non-Heart Failure (HF). Accordingly, “chloride-regaining” diuretics, such as SGLT2i, might preserve vascular volume and induce excessive extravascular fluid to drain into the vascular space via enhanced vascular “tonicity” caused by the increased serum chloride concentration [4]. The “chloride theory” for HF pathophysiology [4] predicts that such a “chloride-regaining” diuretic would not exaggerate the activity of Renin-Angiotensin-Aldosterone System (RAAS) [5]. The mechanisms of SGLT2i treatment for HF via effects on RAAS activity, however, are unclear. The present report describes the case of a patient with T2DM and acute HF who was prescribed diuretic treatment with SGLT2i add-on therapy, in whom specific SGLT2i effects via the “chloride-regaining” diuretic action on the plasma volume and RAAS activity were investigated from the viewpoint of the “chloride theory” [4].
Case presentation
An 85-year-old women, who was treated for T2DM and chronic HF (NYHA functional class III) due to hypertension, pacemaker implantation, and rheumatic valvular heart disease at another hospital, began to visit to my outpatient clinic regularly 1 year prior to the present emergent admission for a chief complaint of progressive dyspnea (NYHA functional class IV) and systemic edema over the preceding 2 weeks. The patient was uneventful for 1 year before the present admission, and she was being treated at the time with a daily dose of glucose-lowering agents (sitagliptin 50 mg and repaglinide 0.5 mg), a blood pressure-lowering agent (amlodipine 5 mg), and diuretics (azosemide 30 mg and spironolactone 25 mg). Her habitus at clinical stability 1 month before the present admission was 143 cm tall and weighing 48.1 kg (body mass index 23.5 kg/m2). She consistently took her prescribed medications. There were no apparent precipitating factors for the present worsening HF event but she was undergoing orthopedic rehabilitation at the time.
Table 1 shows the changes in various clinical tests before and after initiating the SGLT2i (empagliflozin 10mg daily) treatment. Laboratory tests included peripheral blood and urine tests, measurement of B-Type Natriuretic Peptide (BNP), diabetic-related tests, and neurohormonal tests. The background use of glucose-lowering agents, an anti-hypertensive agent, and diuretics described above was kept constant before beginning the SGLT2i treatment and throughout the evaluation period. At the initial presentation of worsening HF (day 1), her blood pressure was 129/84 mmHg, heart rate was irregular at 107 beats/min, temperature 36.5oC, and oxygen saturation 96% on ambient air. Physical examination revealed HF-related physical signs of neck vein distension, severe peripheral edema with weight gain of 1.5 kg over 1 month, and bilateral pulmonary rales. A 12-lead electrocardiogram revealed atrial fibrillation with an irregular heart rate of 107 beats/min and a conducted narrow QRS complex. Cardiac ultrasonography showed preserved left ventricular ejection fraction (54%) and non-dilated diastolic volume (92cc), but a narrowed mitral valve area (0.5 cm2) due to a severely calcified mitral valve and a moderately enlarged left atrium. Doppler echocardiogram showed a moderate degree of tricuspid regurgitation and a raised estimated pulmonary arterial pressure (45 mmHg). Thoracic and abdominal ultrasound revealed moderate pleural effusion and an expanded inferior vena cava with minimal respiratory change. Blood examination revealed an increased serum BNP concentration (from 87 to 203 pg/ml) and HbA1c (9.8% to 10.3%) compared with that at a clinic visit during relative clinical stability 1 month before. Plasma renin activity was markedly enhanced (20.8 ng/mL/h), but plasma aldosterone (267 pg/mL) and anti-diuretic hormone (3.3 pg/mL) levels were only slightly elevated.
Treatment with an SGLT2i (empagliflozin 10 mg daily) was initiated for worsening HF and diabetic derangement. One week later (day 7), the patient’s body weight had markedly decreased by 3.6 kg, and HF-related physical signs and pleural effusion on thoracic ultrasound were ameliorated, but the serum BNP concentration was only slightly reduced. The hemoglobin (+0.5 g/dL) and hematocrit (+0.8%) values changes only slightly from baseline despite marked diuresis, indicating less vascular contraction after SGLT2i administration. The same trends were noted 4 weeks later (day 28).
The serum sodium and chloride concentrations gradually increased on days 7 and 28 under the SGLT2i treatment (sodium from 136 mEq/L before treatment to 137 and 139 mEq/L, and chloride from 100 mEq/L at baseline to 101 and 104 mEq/L, respectively). The serum blood urea nitrogen and creatinine concentrations were mildly increased after treatment. After treatment, plasma renin activity was markedly reduced to 13.1 ng/mL/h on day 7, and further reduced to a near normal level of 3.4 ng/mL/h on day 28. The plasma aldosterone level was reduced to 90.4 pg/mL on day 28 and anti-diuretic hormone levels did not change during the observation period. Two weeks later, the patient was discharged in an acceptable HF status (day 43; Table 1).
Thus, the observations in this patient included preservation of the plasma volume (estimated by monitoring the hemoglobin/hematocrit) and reduced RAAS activity despite the massive diuresis induced by add-on SGLT2i mono-therapy.
Discussion
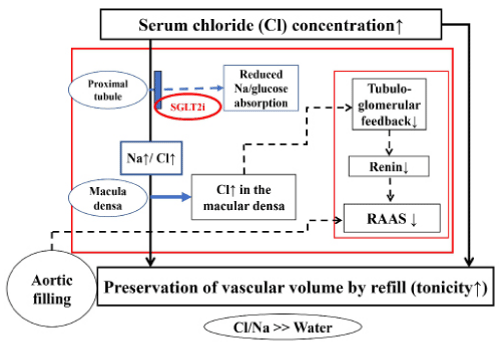
Diuretic treatment with an SGLT2i in a patient with T2DM and acutely decompensated HF lead to three important findings, as follows: SGLT2i diuretic 1) preserved or enhanced the serum chloride concentration, 2) preserved the vascular volume even after massive diuresis, and 3) reduced the exaggerated RAAS activity though RAAS activity is typically enhanced by conventional diuretics [4,5]. Figure 1 summarizes the possible effects caused by SGLT2i on the plasma volume and RAAS activity in the present T2DM/HF patient from the viewpoint of the “chloride theory” [4].
Table 1: Changes in physical and blood tests before and after sodium-glucose transporter-2 inhibitor (SGLT2i) treatment.
Figure 1: Effects of the diuretic action of an SGLT2 inhibitor on the renal tubular Na/Cl and the RAAS according to the “chloride theory”. Solid line indicates enhanced supply or excitatory effect, and dotted line indicates reduced supply or inhibitory effect. Large red square represents the kidney as an action place for the reabsorption of electrolytes and water, and the small red square represents the renin-angiotensin-aldosterone system. Cl: Chloride; Na: Sodium; RAAS: Renin-Angiotensin-Aldosterone System.
SGLT2 inhibitor as a possible “chloride-regaining” diuretic for HF treatment
According to my “chloride theory” for HF pathophysiology [4,5], manipulation of the serum chloride concentration could become an attractive therapeutic target for treating HF [6], such as by reducing the quantity and concentration of serum chloride using conventional diuretics for worsening HF, and preserving and enhancing the concentration of serum chloride with aquaresis using a V2-receptor antagonist [4,7] or acetazolamide [8,9]. On the basis of my recent observations in T2DM/non-HF patients [3], the use of an SGLT2i could be a suitable chloride-regaining diuretic for worsening HF due to its effects to preserve or enhance the serum chloride concentration, as similarly observed in the T2DM patient with acutely decompensated HF reported here. A recent experimental study provided evidence for the chloride-regaining effect of SGLT2i in a rat DM model [10]. Thus, according to the “chloride theory”, diuretic that restores chloride level, such as an SGLT2i, would drain excessive extravascular fluid into the vascular space and preserve vascular volume [11] via enhanced vascular tonicity caused by increased serum chloride concentration in acute decompensated HF [4,5]. The reduced RAAS activity induced by the SGLT2i would favor body fluid reduction in parallel with the osmotic diuretic action.
Diuretic therapy to preserve the serum chloride concentration may induce a residual cardiac volume overload in relation to cardiac function as previously indicated under treatment with a V2-receptor antagonist [4,7] or acetazolamide [8,9]. This concept could apply to the present case undergoing diuretic treatment with an SGLT2i because the vascular volume did not decrease, and thus the magnitude of the serum BNP reduction was so small even after massive diuresis under the SGLT2i diuretic treatment, in accordance with the recent clinical study [12]. Adjusting the combination of various diuretics and their dosages according to their effects on the serum chloride concentration might favorably reduce the vascular volume and contribute to cardiac unloading.
RAAS activity under SGLT2i treatment for worsening HF status
Conventional diuretics typically enhance RAAS activity [5], but in the present case the SGLT2i greatly reduced the RAAS activity (Table 1). The effect of SGLT2i on RAAS activity are controversial [2]. Enhanced RAAS activity could be expected from the effects of blood pressure-lowering and blood vessel contraction caused by the SGLT2i [13]. One report suggested that SGLT2i activates the RAAS by decreasing the chloride supply to the macula densa [14]. Several clinical studies reported enhancement of plasma renin activity [3,15]. Another report, however, indicated that SGLT2i therapy inhibits RAAS activity [16], including some experimental studies [17,18].
In this background, the “chloride theory” [4,5] predicts that SGLT2i therapy would have favoring an inhibitory effect on RAAS activity, as shown in Figure 1. Accordingly, reduced RAAS activity, angiotensin II in particular, may lead to the dilation of efferent glomerular arterioles, thereby reducing glomerular hydraulic pressure, and the glomerular filtration rate. Decreased glomerular hydraulic pressure induced by an SGLT2i would also result from afferent arteriolar constriction via tubuloglomerular feedback [16,19]. Preservation of the serum chloride concentration and plasma volume by the SGLT2i would, however, counterbalance these actions and prevent worsening of renal function [4]. Further studies are needed to investigate RAAS activity in T2DM/HF patients because complex and various changes in the RAAS activity could exist under different phases (e.g., acute, sub-acute, or chronic) of SGLT2i treatment among heterogeneous populations of T2DM patients with HF status of different degree of severity.
Conclusion
In conclusion, a glucose-lowering drug, an SGLT2i, may act as a “chloride-restoring” diuretic with concomitant RAAS activity not to be exaggerated under treatment for worsening HF. Additional studies are required to determine the effects of this agent on the pharmacologic, hemodynamic, neurohormonal, and prognostic aspects of HF pathophysiology in T2DM/HF patients, and the “chloride theory” is expected to provide a background framework for analytic investigation of the diuretic effects of SGLT2i in HF pathophysiology.
Acknowledgements
Appreciation is expressed to Dr Yuichi Yoshida MD for the contribution of collecting clinical data in the present study.
Statement of Ethics
The ethics committee at Nishida Hospital approved the study protocol.
References
- Heerspink HJL, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013; 15: 853-862.
- Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Med Sci. 2019; 20: 629.
- Kataoka H, Yoshida Y. Enhancement of the serum chloride concentration by administration of sodium-glucose cotransporter-2 inhibitor and its mechanisms and clinical significance in type 2 diabetic patients: a pilot study. Diabetol Metab Syndr. 2020; 12: 5.
- Kataoka H. The “chloride theory”, a unifying hypothesis for renal handling and body fluid distribution in heart failure pathophysiology. Med Hypotheses. 2017; 104: 170-173.
- Kataoka H. Rational of the “chloride theory” as an explanation for neurohormonal activity in heart failure pathophysiology: literature review. J Clin Exp Cardiolog. 2019; 10: 634.
- Kataoka H. Proposal for new classification and practical use of diuretics according to their effects on the serum chloride concentration: Rational based on the “chloride theory”. Cardiol Ther. 2020; 9: 227-244.
- Kataoka H. Yamasaki Y. Strategy for monitoring decompensated heart failure treated by an oral vasopressin antagonist with special reference to the role of serum chloride: a case report. J Card Cases. 2016; 14: 185-188.
- Kataoka H. Treatment of hypochloremia with acetazolamide in an advanced heart failure patient and importance of monitoring urinary electrolytes. J Cardiol Cases. 2018; 17: 80-84.
- Kataoka H. Acetazolamide as a potent chloride-regaining diuretic: short- and long-term effects, and its pharmacologic role under the ‘chloride theory’ for heart failure pathophysiology. Heart Vessels. 2019; 34: 1952-1960.
- Masuda T, Watanabe Y, Fukuda K, Watanabe M, Onishi A, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol. 2018; 315: F653-F664.
- Hallow K, Helmlinger G, Greasly PJ, McMurray JJ, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization?: A differential volume regulation hypothesis. Diabetes Obes Metab. 2018; 20: 479-487.
- Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TD, et al. Randomized, double-blind, placebo-controlled, multicenter pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020; 22: 713-722.
- Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016; 134: 752-772.
- Kimura G. Importance of inhibiting sodium-glucose cotransporter and its compelling indication in type 2 diabetes: pathophysiological hypothesis. J Am Soc Hypertens. 2016; 10: 271-278.
- Higashikawa T, Ito T, Mizuno T, Ishigami K, Kohori M, et al. Effects of tofogliflozin on cardiac function in elderly patients with diabetes mellitus. J Clin Med Res. 2020; 12: 165-171.
- Gilbert RE. Sodium-glucose linked transporter-2 inhibitors: Potential for renoprotection beyond blood glucose lowering? Kidney Int. 2014; 86: 693-700.
- Mori I, Ishizuka T. Effects of SGLT2 inhibitors on renin-aldosterone system for one month and six months in type 2 diabetes. Diabetes. 2018; 67. (Suppl. 1): https://doi.org/10.2337/db18-1196-P.
- Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, et al. Effects of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE. 2016; 11: e0165703.
- Vallon V, Miracle C, Thomson S. Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail. 2008; 10: 176-187.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.