Annals of Cardiology and Vascular Medicine
HOME /JOURNALS/Annals of Cardiology and Vascular Medicine- Review Article
- |
- Open Access
Heart failure with preserved ejection fraction (HF-PEF): A mini-review
- Peysh A Patel;
- Leeds General Infirmary, UK
- Noman Ali
- Leeds General Infirmary, UK

| Received | : | Dec 20, 2017 |
| Accepted | : | Mar 19, 2018 |
| Published Online | : | Mar 26, 2018 |
| Journal | : | Annals of Cardiology and Vascular Medicine |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Patel PA; Ali N. Heart failure with preserved ejection fraction (HF-PEF): A mini-review. Ann Cardiol Vasc Med. 2017; 1: 1002.
Abstract
Heart Failure with Preserved Ejection Fraction (HF-PEF) describes patients with classic symptoms but without left ventricular systolic impairment (ejection fraction ≥ 50%). These cohorts have features of diastolic dysfunction which is considered a precursor and associated with poor outcomes. HF-PEF appears to be of increasing prevalence, which may relate to an ageing population as well as improved recognition of the disorder. It is prudent to explore underlying aetiology, which is usually a primary myocardial disorder. Echocardiography is the cornerstone for diagnosis, though interpretation is often limited if there is co-existent Atrial Fibrillation (AF). Currently, no established guidelines exist to direct management with disappointing results from clinical trials. Therapy is primarily targeted at alleviating symptoms of congestion and treating potential precipitants.
Keywords: Heart failure; Congestive heart failure; Primary myocardial disorder; Cardiac imaging
Definitions
Classically, Congestive Heart Failure (CHF) is seen as a clinical syndrome with presence of typical symptoms and signs and primarily attributable to Left Ventricular Systolic Dysfunction (LVSD) [1]. As per European Society of Cardiology guidelines, diagnosis is confirmed by cardiac imaging with an arbitrarily defined Ejection Fraction (EF) <40% [2]. The last three decades has seen a paradigm shift in our understanding of the condition, triggered by the observation that structural and functional perturbations can precede clinical manifestations and be associated with adverse outcomes. The term ‘Heart Failure with Preserved Ejection Fraction’ (HF-PEF) has been coined to describe this phenomenon, and encompasses cohorts with suggestive clinical features of CHF but in the context of preserved systolic function (EF ≥ 50%) and without attributable valvular disease. It can also loosely be referred to as ‘diastolic heart failure’. Moreover, there is now a ‘grey area’ defined as HFmrEF for those with LVEF of 40-49%, who are likely to have attributable mild systolic dysfunction but with some features of diastolic impairment.
Epidemiology
The burden of CHF in the UK is increasing, and comparable to the cumulative impact of the four most common types of malignancy [3]. In the community setting, approximately half of all patients with clinical features of CHF have preserved EF on objective assessment [4]. Epidemiological studies have observed that prevalence increases with age, with a higher predilection in females [5]. The risk across ethnic groups has not been well characterised. Hypertension is the most discernible risk factor for the development of HF-PEF [6]. Additional precipitants in clude diabetes mellitus, obesity and history of coronary artery disease. Generally, multi-morbidity is more common in patients with HF-PEF, with around 50% having five or more [7].
Pathophysiology
A number of mechanisms have been implicated in the development of HF-PEF, including impaired ventriculo-arterial coupling, chronotropic incompetence and endothelial dysfunction [8]. However, the most significant contributor is deemed to be diastolic dysfunction, a feature that typically co-exists with, and often precedes, LVSD. Diastolic dysfunction results in an elevation of Left Ventricular End-Diastolic Pressure (LVEDP) and thus left atrial pressure. This may cause pulmonary congestion and symptoms of breathlessness can arise as a consequence.
Given the close relationship between HF-PEF and diastolic dysfunction, an understanding of the physiology of diastole is critical to comprehend the pathophysiology that underpins HF-PEF. Diastole is typically defined as the period between the closure of the aortic valve (end-systole) and the mitral valve (end-diastole), and can be divided into four distinct periods: isovolumic relaxation, early rapid diastolic filling (‘E’ wave), diastasis and late diastolic filling (‘A’ wave) [9]. These phases can be subcategorised into two groups: active myocardial relaxation which encompasses the first two phases, and passive ventricular filling which encompasses the final two.
As the name implies, active myocardial relaxation requires production of Adenosine Triphosphate (ATP) from within the myocardium. During isovolumic relaxation, left ventricular pressure falls following aortic valve closure but without a change in volume. When the pressure falls below the atrial pressure, the mitral valve opens. This defines commencement of the early rapid diastolic filling phase with blood filling the left atrium from the pulmonary veins and flowing across the valve into the left ventricle (‘E’ wave). The flow rate is influenced by several parameters including pressure gradient, ventricular relaxation and ventricular compliance. Left ventricular compliance is a passive feature that may be affected by myocardial characteristics such as hypertrophy, or extrinsic factors such as constrictive pericarditis. With the flow of blood, there is gradual equalisation of pressures between atrium and ventricle, resulting in a period of diastasis during which there is minimal flow. This duration is longer at slower heart rates. The final phases of diastole arise as a result of atrial contraction, which transiently increase left atrial pressure and cause a period of late diastolic filling (‘A’ wave). The phases of diastole in the right ventricle are analogous to those described above, other than with regards to total duration which is shortened due to a longer systolic ejection period.
Diagnosis and assessment
As alluded to, subcohorts may have precursor features pertaining tostructural and functional abnormalities but be entirely asymptomatic. This makes the diagnosis of HF-PEF particularly challenging. Nonetheless, the initial step requires identification for presence of symptoms and/or signs of CHF, such as dyspnoea, orthopnoea and peripheral oedema. Biochemical profiling for elevated BNP (> 35 pg/ml) or NT-proBNP (> 125 pg/ml) levels is also necessitated as part of the diagnostic work-up [2]. However, it is most relevant for its Negative Predictive Value (NPV) and therefore is particularly beneficial in formally excluding the diagnosis when index of suspicion is low. Moreover, interpretation is confounded by intrinsic rhythm, particularly Atrial Fibrillation (AF), and lacks specificity as values can be raised in the context of a wide range of both cardiac and non-cardiac causes [10]. A 12-lead Electrocardiogram is also routinely performedto establish rate, rhythm, QRS morphology and potential indicators of aetiology, though it lacks specificity.
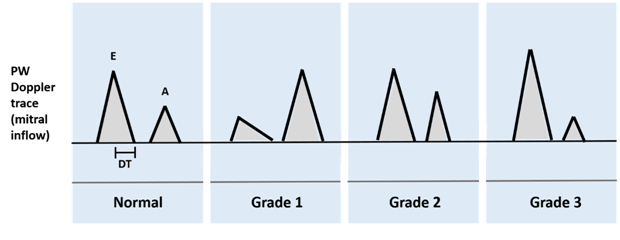
After these initial assessments, evidence of a preserved EF and absence of significant valvular disease must be sought. Echocardiography is the most commonly used diagnostic tool for this purpose, and has the benefit of allowing an assessment of left ventricular diastolic function. This is done via measurements of Pulsed Wave (PW) Doppler flow across the mitral valve. A classification of diastolic dysfunction based upon echocardiographic parameters has been developed, and broadly speaking, three grades are described (Figure 1) [11].
In grade 1 diastolic dysfunction, slower ventricular relaxation results in a reduced rate of decrease in ventricular pressure. This delays opening of the mitral valve and reduces the transmitral gradient, resulting in a prolonged Isovolumic Relaxation Time (IVRT), reduced E wave, prolonged Deceleration Time (DT) and increased A wave (due to compensatory filling of atrium). Classically, this manifests as a reversed E: A ratio (i.e. height of A wave > E wave). In Grade II (pseudo-normal) diastolic dysfunction, there is a transition in pathophysiology from abnormal relaxation alone to an impairment of relaxation and compliance. Thus, the E velocity is normal due to a concurrent increase in left atrial pressure which drives flow across the valve. Additionally, there is normalisation of the DT as the decreased compliance results in a rapid rise of ventricular pressure during early diastole. Detection of grade II diastolic dysfunction is via Tissue Doppler Imaging (TDI) to assess E / annular E’ ratios, as well as identification of flow reversal into pulmonary veins, Valsalva manoeuvres to transiently reduce LA pressure and objective identifications of ancillary findings such as left ventricular hypertrophy or atrial dilatation. Grade III diastolic dysfunction represents a restrictive filling pattern, whereby relaxation and compliance are still impaired but the compensatory increase in left atrial pressure masks underlying abnormalities. Thus, a shortened IVRT arises (due to earlier opening of mitral valve) with increased E/A ratio, shortened DT and reduced/absent A wave with little filling because of the raised LVEDP
The American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) have provided a recent updated criteria for assessment of diastolic function [12]. Presence of elevated left ventricle (LV) filling pressures is specifically advocated as the first step in determining severity. The four recommended variables to utilise are annular E’ velocity, average E/E’ ratio, Left Atrium (LA) volume index and peak tricuspid regurgitation velocity. Dysfunction is considered to be present if over 50% of these parameters exceed recommended cut-off values.
In cases where Echocardiography is unable to provide diagnostic certainty, such as in the context of AF, invasive assessment of left ventricular filling pressure remains the gold standard modality of choice. Further testing may be indicated if HF-PEF is deemed to be secondary to a reversible cause, such as poorly controlled hypertension or myocardial ischaemia. In these circumstances, Ambulatory Blood Pressure Monitoring (ABPM) or coronary angiography may be of adjunct benefit.
Management
Observational studies have shown HF-PEF to be associated with similar mortality rates to LVSD [5,13]. However, whereas a number of therapies have been demonstrated to provide a mortality benefit in LVSD, the same is not true of HF-PEF; angiotensin-converting enzyme (ACE) inhibitors, Angiotensin II Receptor Blockers (ARBs) and beta-blockers have all failed to improve outcomes in patients with HF-PEF [14]. Initial promise was demonstrated with use of aldosterone antagonists amongst patients with mild HF-PEF, but a subsequent trial failed to replicate this in a cohort of patients with more advanced disease [15]. The paucity of evidence-based therapy has meant that a lack of clarity persists about how best to manage these subcohorts. European Society of Cardiology (ESC) guidelines currently advocate that the primary focus of management is placed on rigorous identification and treatment of co-morbidities, such as hypertension, diabetes mellitus and obesity [2]. Diuretic therapy is often indicated for symptomatic relief, but caution needs to be exercised to avoid excessive lowering of preload. It is additionally of no prognostic benefit.
Conclusion
HF-PEF has rapidly become a focus of attention amongst cardiologists due to persistently poor outcomes for patients diagnosed with the condition. The rising prevalence of HF-PEF suggests that it will continue to be of significant public health burden, and this highlights the pivotal importance of further research in this field to fill the remaining gaps that currently exist in our collective knowledge base.
References
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251–259.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891-975.
- Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, et al. Temporal trends and patterns in heart failure incidence: a populationbased study of 4 million individuals. Lancet. 2017; S0140-6736: 32520-32525.
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017; 14: 591-602.
- Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009; 119: 3070-3077.
- Teo LY, Chan LL, Lam CS. Heart failure with preserved ejection fraction in hypertension. CurrOpinCardiol. 2016; 31: 410-416.
- Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015; 128: 38-45.
- Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014; 115: 79-96.
- Kapila R, Mahajan RP. Diastolic dysfunction. Continuing Education in Anaesthesia Critical Care & Pain. 2009; 9: 29–33.
- Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW. Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. J Card Fail 2011; 17: 729-734.
- Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart. 2005; 91: 681-695.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29: 277-314.
- Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008; 1: 91-97.
- Ao A, Shah SJ. Diagnosis and Management of Heart Failure with Preserved Ejection Fraction: 10 Key Lessons. Current Cardiology Reviews. 2015; 11: 42-52.
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2014; 370: 1383-1392.