- Research Article
- |
- Open Access
Risk Assessment-Based Breast Cancer Screening Programs: A U.S. Environmental Scan
- Susan R Snyder*;
- Georgia State University, School of Public Health, Atlanta, GA, USA.
- Dina Hassen;
- Geisinger, Danville, PA, USA.
- Alanna Kulchak Rahm;
- Geisinger, Danville, PA, USA.
- Jing Hao;
- Geisinger, Danville, PA, USA.
- Samantha Crissinger;
- Geisinger, Danville, PA, USA.
- Qiang Hao;
- Geisinger, Danville, PA, USA.
- Mallory Snyder;
- Geisinger, Danville, PA, USA.
- Rosemary Leeming
- Geisinger, Danville, PA, USA.

| Received | : | Nov 01, 2020 |
| Accepted | : | Feb 01, 2021 |
| Published Online | : | Feb 03, 2021 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Snyder SR, Hassen D, Rahm AK, Hao J, Crissinger S, et al. Risk Assessment-Based Breast Cancer Screening Programs: A U.S. Environmental Scan. Ann Breast Cancer. 2021; 4(1): 1016.
Abstract
Objective: A paradigm shift has occurred in evidence-based breast cancer screening and prevention recommendations towards a personalized risk assessment-based approach. A resulting trend is dramatic growth in new High Risk Breast Clinic (HRBC) programs incorporating a front-end risk assessment. There is a lack of standardized information on the implementation of these emerging programs which are characterized by substantial variation. The objective of this study is to collect and synthesize information describing the current state of the U.S. national landscape of HRBCs, and to develop a conceptual framework for evaluating these programs.
Methods: A national environmental scan based on a survey of HRBC programs combined with a review of literature and publicly available information used systematic methods to identify programs and develop a conceptual framework and data collection instrument based on key program characteristics. Results analysis uses descriptive statistics to report on the variability in attributes organized by several domains to identify differences between HRBC programs.
Results: Information from more than 100 U.S. HRBC programs is synthesized using a conceptual framework of attribute domains. Analysis of program attributes summarizes variation within the following categories: target population, access criteria, structure, staff composition, coordination of services, risk assessment methods, services, workflow, implementation barriers and facilitators, and use of outcome measures.
Conclusions: The environmental scan findings and conceptual framework are important first steps in describing HRBC programs which can be used to inform and facilitate their development, implementation and evaluation using a standardized approach. Future work and implementation can lead to improvements in the quality and delivery of personalized, evidence-based breast cancer screening and prevention care provided to women of all risk levels and contribute to optimizing breast cancer screening outcomes.
Introduction
Breast cancer is the most common type of cancer in the U.S. with 279,100 new cases expected in the United States in 2020 [1]. While routine mammography screening is associated with early detection and a reduction in death rates from breast cancer [2], it is also associated with a larger than is generally recognized rate of overdiagnosis, or the diagnosis of a “cancer” that would otherwise not cause symptoms or death, [3,4] and overtreatment [5]. The national burden of breast cancer overdiagnosis and overtreatment is estimated at $4 billion annually [6].
There has been growing evidence-based consensus supporting the inclusion of breast cancer risk assessment in routine care to deliver personalized risk-based screening, representing a paradigm shift away from one-size-fits-all screening [7]. Risk-based screening is considered more effective at achieving population health and patient-centered care objectives of shared decision making, accurate and timely diagnosis [8], appropriate use of breast cancer screening practices, including MRI and referrals for genetic counseling, and reducing inappropriate screening that disproportionately causes overdiagnosis and overtreatment [9].
While there is some variation among national guideline-issuing organizations (US Preventive Services Task Force (USPSTF) [10], American Cancer Society (ACS) [11], American College of Radiology (ACR) [12], American College of Obstetrics and Gynecology (ACOG) [13]) on the specific timing and frequency of breast cancer preventive screening, they increasingly support risk-stratified approaches. Current evidence-based guidelines recommend different screening strategies for different levels of breast cancer risk, which can be quantified through various risk assessment models [14] (e.g., Gail [15], Claus [16]).
To implement the recent proliferation of risk factor-based guidelines and requirements for breast cancer screening and prevention, there has been substantial growth in new “high risk breast clinic” (HRBC) programs. According to a 2015 survey of 78 Oncology Roundtable members, almost 30 percent already had a high risk breast clinic and 36 percent planned to develop an HRBC within two years [17]. HRBC programs providing individualized multidisciplinary care emerged as a means for delivering personalized evidence-based healthcare beginning with risk assessment and personalized screening and prevention strategies followed by the continuum of care subsequent to a breast cancer diagnosis. Programs offer coordinated care which may include clinical examinations, breast imaging/radiology, risk counseling and education, genetic counseling and testing, care navigators, oncology services, support groups, and clinical trials [18,19]. These programs, however, are characterized by substantial variation [19].
While there are a number of articles describing individual programs in the literature which we used to identify programs as well as inform our conceptual framework, currently there is very limited information available describing the variation in implementation approaches of risk assessment-based, individualized care for breast cancer screening and prevention, and no effectiveness evidence. The objective of this study is to collect and synthesize information describing the current state of the U.S. national landscape of HRBCs, and to develop a conceptual framework for evaluating these programs.
Methods
Search strategy
A search strategy was used to achieve two main objectives: 1) identify HRBC programs to survey, and 2) identify program attributes based on publicly available information to develop a conceptual framework to generate the survey domains and responses. We applied the following three characteristics as inclusion criteria to the search results to identify organizations eligible for the survey: 1) a front-end individual breast cancer risk assessment process based on at least one validated risk assessment method; 2) patients’ breast cancer risk assessment results used in conjunction with guideline-based recommendations for screening and prevention services; and 3) U.S.-based program. The search for programs was conducted using multiple modes: 1) a literature search of PubMed and Medline databases, 2) a web-based search, and 3) professional contacts. The web-based search was used to identify programs through their websites or the websites of affiliated organizations, and additional programs were identified through project team members’ professional contacts, including the National Society of Genetic Counselors.
Survey development
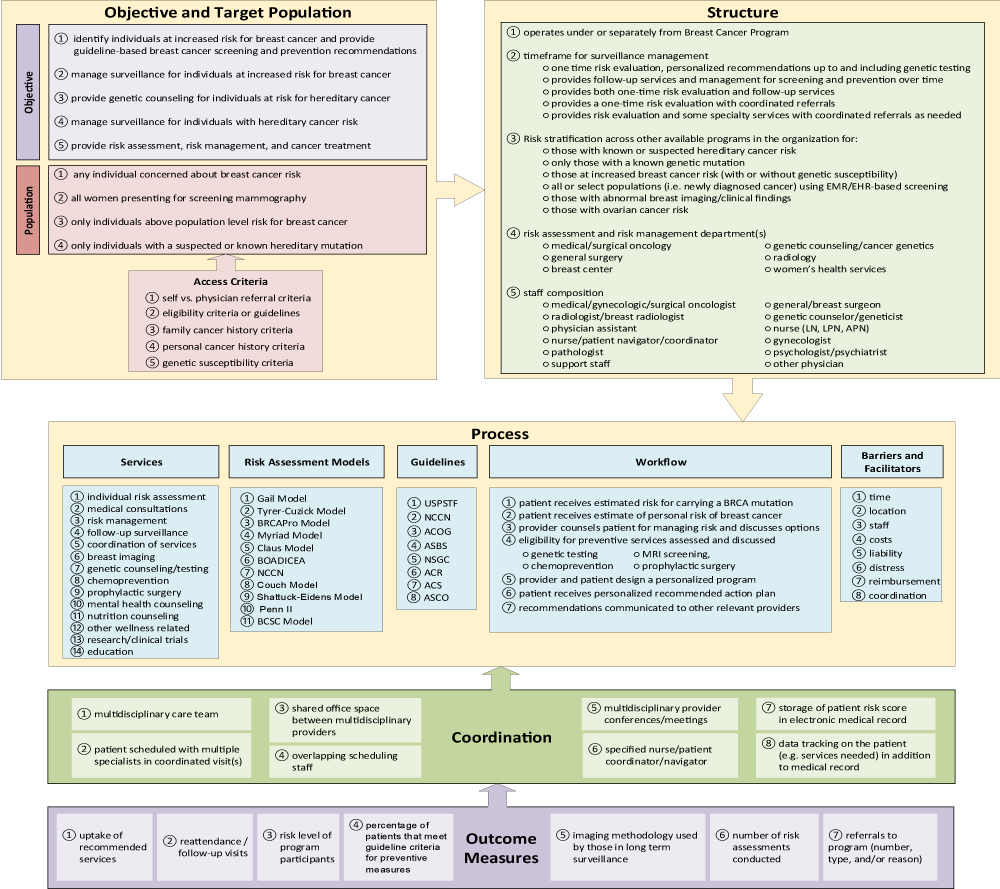
Profiles of each HRBC program identified were created using publicly available information from their websites and/or available literature. Using these profiles and additional publicly available information, a conceptual framework was created to identify program domains and their corresponding attributes to serve as a framework for developing the survey (Figure 1). The survey underwent multiple iterations and was reviewed by project team members and clinicians, and pilot tested by genetic counselors working at Geisinger’s High Risk Breast Clinic and Inherited Risk Clinic. The project team also consulted with Geisinger’s Survey Research and Recruitment Core (Survey Unit) on the survey design and dissemination methods.
Survey dissemination
Information collected during the compilation of the HRBC program profiles included contacts with all potentially relevant individuals within the programs such as program coordinators, program directors, breast surgeons, etc., who would be appropriate survey respondents. A research assistant also contacted each of the programs identified by phone and/or fax to describe the research and request information on their personnel who would be best qualified to respond to the survey. In cases where multiple individuals were identified, the survey was sent to more than one person to increase the likelihood of a positive response. In addition, if there was a relationship between a program contact and a project team member, they made direct contact and provided the survey link. If a program was identified from the literature, the corresponding author was contacted. REDCap [20] was used for administering the survey during 2018 which was sent by email and/or fax with a cover letter to the identified contacts with an individual survey web-based link.
Results
HRBC program search
Overall, of the 130 programs identified, we were able to obtain at least one email address of an appropriate contact for 60 of the programs, and a fax number alone for an additional 20 programs, for a total of 80 programs contacted. Sixteen programs responded, for an overall response rate of 20 percent (27 percent for programs contacted by email only). Table 1 details the search strategy results and the programs identified.
Demographic characteristics (domain I)
Responding programs were evenly distributed across four geographic regions (Northeast, Midwest, South, and West). A substantial proportion of programs (44 percent) served less than 100 patients per month (range: 20 - 5,800). The program with the highest volume indicated they perform risk assessments on anyone presenting for any kind of imaging. One-half of the programs saw between 5 and 80 new patients per month. One-fourth of the programs with 100 to 400 new patients per month were all based in Genetic Counseling/Cancer Genetics departments.
Objective and population (domain II)
Program objective
Most programs responding aimed to identify women at increased risk. Well over half the programs aimed to provide a range of care for women at increased risk for breast cancer including managing surveillance, providing genetic counseling for any hereditary cancer, providing care for those with hereditary risk, and providing risk assessment, risk management and cancer treatment.
Target population
The programs responding were split between targeting all women/any women concerned about risk (56 percent) versus only those with increased risk (44%). Other notable target population information obtained from publicly available information included women with abnormal biopsy results and women with noninvasive breast cancer that had been treated with surgery or radiation therapy. Individuals with above population-level risk for breast cancer may include those with a strong personal or family history of breast or any cancer. One-fourth of the programs responding specifically targeted underserved and/or minority women at risk for breast cancer.
Program access criteria
Most of the surveyed programs did not require a physician referral, and almost all of the programs accepted self-referrals. However, almost 40 percent had referral guidelines based on definitions for a high-risk population. Of these, 40 percent had family cancer history related guidelines, 30 percent had some type of personal cancer history guidelines, and almost one-third had guidelines relating to personal or family genetic susceptibility to cancer. About one-fourth of the programs had referral guidelines based on risk assessment scores. Other notable variations in program access criteria found from publicly available information included staff obtaining prior authorization to determine whether the patient meets criteria prior to scheduling a visit.
Structure (domain III)
Department affiliation
Most programs responding operated under a Breast Cancer Program/Clinic. Almost 40 percent of the programs were based in Genetic Counseling/Cancer Genetic departments, with another 40 percent of the programs distributed between Oncology/Surgical Oncology, General Surgery, and Radiology. The remainder of the programs were based under a Breast Center, Women's Health Services, or under multiple departments (Breast Surgery and Radiology).
Program care management timeframe
Most of the programs responding provided follow-up services and management of screening and prevention over time. Less commonly reported approaches included providing a one-time risk evaluation with personalized recommendations up to and including genetic testing, providing both one-time risk evaluation and follow-up services, providing a one-time risk evaluation with coordinated referrals, and providing risk evaluation and some specialty services with coordinated referrals as needed.
Related programs in organization
Over half of the respondents indicated their organizations offer a program specific to patients with known genetic mutations, and most had programs for women at increased breast cancer risk, with or without genetic susceptibility.
Staff composition
The majority of programs responding to the survey were led by a General/Breast Surgeon or Breast Surgical Oncologist, followed by a Genetic Counselor/Geneticist. Oncologists (Medical/Gynecologic/Surgical) were the leading specialty reported as core program staff, followed by Genetic Counselor/Geneticists, and General/Breast Surgeons. All specialties included in the core program staff are listed in Table 2.
Coordination (domain IV)
At least half the responding programs reported using the following to facilitate coordination: 1) scheduling patients with multiple specialists in coordinated visit(s); 2) having multidisciplinary provider conferences/meetings; 3) performing data tracking on patient (e.g. services needed) in addition to medical record; and 4) storing patient risk score in electronic medical record (almost 90 percent). Having a delegated role for coordination was also noted in program profiles as well as in the literature [21]. As shown in Table 2, a multidisciplinary team is a key component of coordination with almost 40 percent of programs having a nurse/patient navigator. Additional elements of coordination and communication both with patient and provider(s) are detailed under Risk Assessment Workflow (Table 4).
Risk assessment, models, and guidelines (domain V)
Risk assessment
Genetic Counselors performed the risk assessment in over half of the programs responding to the survey, followed by nurse practitioners/advanced practice registered nurses/oncology certified nurses, physicians or surgeons, and physician assistants. About one-third of the programs had multiple types of providers perform the risk assessment. Programs obtain the patient intake information via a variety of methods including a questionnaire administered by paper and/or electronically prior to or during an appointment, and/or recorded by provider/staff via discussion during an appointment. Two programs noted that a genetic counselor contacts the patient prior to the visit to collect family history information.
Models
Programs were asked about the types of models used to conduct risk assessments and to note whether the models were primary, secondary, and/or taken into consideration. All programs responded that multiple models were used. Tyrer-Cuzick was the most common primary model (almost 70 percent), followed by the Gail model and the questionnaire based on NCCN Guidelines which each had 38 percent. In terms of software, half of the programs utilized either Cancer Gene software or Hughes riskApps (CRA Health). Other models considered primary or secondary were BRCAPro, Claus, modified Gail allowing for incorporation of other factors, Myriad, and BOADICEA.
Guidelines
All programs responding considered multiple guidelines. The National Comprehensive Cancer Network (NCCN) guidelines were the most commonly reported among the primary guideline(s) used. Other guidelines included: U.S. Preventive Services Task Force (USPSTF), American College of Obstetricians and Gynecologists (ACOG), American Society of Breast Surgeons (ASBS), National Society of Genetic Counselors (NSGC), American College of Radiology (ACR), and The American Cancer Society (ACS).
Services (domain VI)
The services offered by the programs responding are listed in Table 3, and the majority are conducted by the core program (or administered by core program staff) or via referrals.
Risk assessment workflow (domain VII)
The post-risk assessment evaluation and consultation processes performed by the majority of respondents are detailed in Table 4. Half of the respondents reported multiple types of providers performing the risk management consultation. The most common type of provider performing the risk management consultation is a nurse practitioner (50%), followed by a genetic counselor (38%), physician (31%), and surgeon including breast surgeon, or surgical oncologist (25%).
Barriers and facilitators (domain VIII)
Barriers
The most commonly reported barriers affecting HRBC program operations were insurance reimbursement not covering the total costs of program services followed by a lack of insurance coverage for genetic testing/other services for high risk patients. Barriers also included program costs (e.g., staff salaries/training expenses). Other barriers identified from the initial search included: implementing screening and prevention recommendations across multiple disciplines, lack of knowledge by other providers on latest guideline recommendations, need for revenue generation, tracking downstream revenue generation, providing resources (e.g. training time), patient concern over the potential for insurance discrimination (e.g. loss of health insurance), low participation rate, and potential exclusion of increased risk patients due to eligibility criteria.
Facilitators
The most commonly reported program facilitator was "appropriate physical location," followed by "appropriate specialized staff." Facilitators also included "sufficient amount of time to conduct risk evaluation and counseling." Other facilitators included: coordinating staff schedules across disciplines (e.g., having designated times with providers from multiple disciplines at the program/clinic), coordination/streamlining patient visits and care by designated staff (e.g., navigator), standardizing all reports/letters/documentation (e.g., to other healthcare providers), raising awareness of the program, efficient risk assessment process, updating other providers on latest guideline recommendations, community lectures/education on genetic counseling, and physician involvement/interaction with patients to improve adherence to screening and prevention guideline recommendations.
Measurement/data (domain IX
)Almost half of the survey respondents indicated their program records and tracks or records outcome measures as detailed in Table 5.
Discussion
This environmental scan of U.S. programs implementing risk assessment-based breast cancer screening addresses an important knowledge gap concerning how the shift in evidence-based guidance to personalized, risk-based screening is being translated into practice. A personalized risk assessment-based approach to breast cancer screening offers the potential to be more effective and efficient at delivering beneficial preventive care to women of all risk levels, and importantly is likely to perform better at identifying high-risk women who may be offered more targeted screening and risk reduction services. As more evidence for breast cancer risk stratification, screening and prevention continues to emerge, a risk assessment-based screening model such as High Risk Breast Clinic (HRBC) programs is likely better prepared to incorporate new findings and implement updated recommendations.
This is the first study to: 1) describe and categorize the attributes of HRBC programs, and 2) compare characteristics across surveyed programs. The approach employed relied on both survey responses and publicly available information on identified programs, which were used to develop a conceptual framework. The primary limitation of this study is the small number of survey respondents. The detailed nature of the survey may have contributed to a lower response rate. Nonetheless, this study provides an important first step in identifying program characteristics and domains, and in assessing variations in key elements including differences in implementation. Dissemination of these results may be useful to existing programs as well as to organizations developing new programs by providing information that may facilitate implementation on a larger scale.
Although HRBC programs appear to offer a promising approach for implementing risk-assessment based screening and preventive services, they do not yet constitute a defined model, and further work is needed to standardize and provide information characterizing these programs, their variations, and ultimately evaluating their performance. The results of this initial environmental scan of surveyed programs and publicly available information is a first step. The development of a formalized care model can aid in evaluation of these programs, which offer the potential for improved adherence to effective breast cancer screening and preventive care as well as increased use of shared decision making. Current non-HRBC approaches to breast cancer screening do not yet routinely integrate individualized, risk assessment, which is also impeded by a lack of consistency in breast cancer screening guidelines. This study’s results demonstrate that HRBC program efforts to improve uptake of risk assessment tools linked to evidence-based breast cancer screening and prevention practices can facilitate implementation of a personalized risk-based approach, and offer the potential to improve outcomes for women of all risk levels.
References
- National Institutes of Health, National Cancer Institute Common Cancer Types. https://www.cancer.gov/types/common-cancers. Accessed November 19, 2020.
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005; 353: 1784-1792.
- Welch HG, Black WC. Over diagnosis in cancer. J Natl Cancer Inst. 2010; 102:605-613.
- Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med. 2016; 375: 1438-1447.
- Hubbard RA, Kerlikowske K, Flowers CL, Yankaskas BC, Zhu W, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011; 155: 481-492.
- Ong MS, Mandl KD. National Expenditure for False-Positive Mammograms and Breast Cancer Over diagnoses Estimated At $4 Billion a year. Health Affairs. 2015; 34: 576-583.
- Onega T, Beaber EF, Sprague BL, Barlow WE, Haas JS, et al. Breast cancer screening in an era of personalized regimens: A conceptual model and National Cancer Institute initiative for risk‐based and preference‐based approaches at a population level. Cancer, 2014; 120: 2955-2964.
- Depypere H, Desreux J, Pérez-López FR, Ceausu I, Erel CT, et al. EMAS position statement: Individualized breast cancer screening versus population-based mammography screening programmes. Maturitas. 2014; 79: 481-486.
- Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Over diagnosis, and Mammography Screening Effectiveness. New England Journal of Medicine. 2016; 375: 1438-1447.
- Siu AL. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2016; 164: 279-296.
- American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Accessed November 19, 2021.
- Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. Journal of the American college of radiology. 2010; 7: 18-27.
- American College of Obstetricians and Gynecologists. ACOG statement on breast cancer screening guidelines. 2016.
- Depypere H, Triosi R, Broecke RVD, Vertriest C, Dierickx A, et al. Surgery in metastatic breast cancer. 2014.
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute. 1989; 81: 1879-1886.
- Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991; 48: 232-242.
- Oncology Roundtable, High-Risk Breast Clinics Excerpt from Tumor Site Centers of Excellence. 2015.
- Calzone KA, Stopfer J, Blackwood A, Weber BL. Establishing a cancer risk evaluation program. Cancer practice. 1996; 5: 228-233.
- Oncology Roundtable 2015.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009; 42: 377-381.
- Morman NA, Byrne L, Collins C, Reynolds K, Bell JG. Breast Cancer Risk Assessment at the Time of Screening Mammography: Perceptions and Clinical Management Outcomes for Women at High Risk. Journal of genetic counseling. 2017; 26: 776-784.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.