- Review Article
- |
- Open Access
Pro-necroptotic agents in cancer therapy
- Ahmed El-Sharkawy;
- Human Molecular Genetics Laboratory, Institute of Genetics and Biophysics “A. Buzzati-Traverso” (IGB)-CNR, Naples, Italy
- Biomolecular Science Programme, Università DegliStudi Della Campania “Luigi Vanvitelli”, Naples, Italy
- Ahmed Malki
- Biomedical science Department, College of Health Sciences, Qatar University, Doha, Qatar

| Received | : | Apr 26, 2018 |
| Accepted | : | Oct 12, 2018 |
| Published Online | : | Oct 19, 2018 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: El-Sharkawy A, Malki A. Pro-necroptotic agents in cancer therapy. Ann Breast Cancer. 2018; 1: 1004.
Introduction
Cell survival must exist in equilibrium with cell death in order to maintain proper organ development and cellular and tissue homeostasis [1]. Several forms of cell death have been discovered and well characterized during the last years. One of the best studied form of cell death is apoptosis, which can be defined as an ordered and controlled way to program a cell to die. Apoptosis is essential for normal tissue turnover, development, differentiation and immune responses [2-4]. In apoptosis, a set of morphological features occur including chromatin condensation, nuclear fragmentation, cell shrinkage, plasma membrane blebbing and the formation of an apoptotic bodies. In contrast to the planned cell death in apoptosis, necrosis is another form of accidental cell death that results in a massive death of cells and disruption of normal homeostasis. It occurs upon exposure of cells to severe and overwhelming stresses like toxic compounds or high dose radiation. Recently, another form of ordered necrosis termed necroptosis has been described. This form of cell death servesa central roles in development, cancer pathology, immunity and degenerative diseases [5-8]. It is regulated by receptor interacting protein kinase-1 (RIPK1), RIPK3, and mixed lineage kinase domain-like (MLKL) [9-11].
Mechanisms
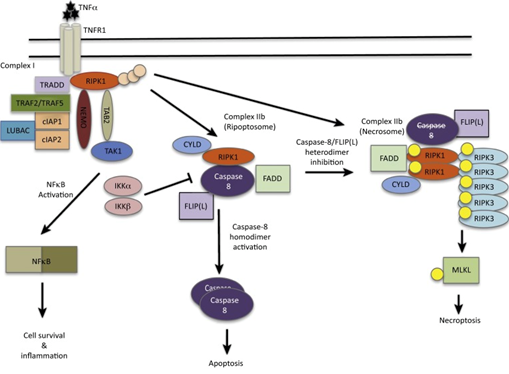
Several death receptors can induce necroptosis: Fas, TRAIL1, TRAIL2 and TNFR-1. These receptors trigger specific pathogen recognition receptors like: TLR3 & 4 and DAI (the z-DNA sensor DNA-dependent activator of IFN-regulatory factors). Also they can activate T-cell receptors and IFNs type I and II. The binding of TNF to TNFR1 represents one of the best studied signaling pathways of necroptosis. This binding induces the formation of several protein complexes which involved in pro-inflammatory and survival signaling (complex I), apoptosis (complex IIa and IIb) and necroptosis (complex IIc) [12-14]. Necroptosis induction by TNFR1 triggering is activated only when apoptosis signaling is blocked such as in down regulation or inhibition of caspase-8 or inhibitors of apoptosis [15]. Figure 1 [16].
For initiation of necroptosis, deubiquitylated RIPK1 binds to RIPK3 through RHIM domains (RIP homotypic interaction motif). This interaction leads to oligomerization of RIPK3 and its subsequent activation. Followed the activation of RIPK3 through phosphorylation, another effector protein called MLKL is activated and translocated to plasma membrane.
How MLKL is recruited into plasma membrane remains an enigma. Intense investigations carried out to explain the mechanisms by which MLKL is recruited to plasma membrane and executes death of the cell. Some studies showed that translocation of MLKL either to plasma membrane or to intracellular membranes involved with interaction PIPs (phosphatidylinositol phosphates) followed by oligomerization [17,18]. Shifting of MLKL from complex IIc to membranes employs lipids as essential components as PIPs, including PI(5)P and PI [4,5] P2, are required for MLKL membrane targeting. This was depicted as liposomes containing PI but not PIPs don’t result in MLKL-dependent leakage [18]. Moreover, interfering with the formation of PI(5)P or PI [4,5] P2 inhibits necroptosis but not apoptosis [17].
Natural products as pro-necroptotic agents in cancer therapy
A growing list of several natural products with distinct mechanisms of actions to employ necroptosis as a trigger of cell death were described. Of which, Shikonin, which is a naphthoquinone naturally occurring compound, triggers cell death with morphological features consistent with necroptosis that was inhibited in presence of Necrostatin-1 [19].
Shikonin exhibited a comparable potency toward drug-sensitive cancer cell lines as well as their drug-refractory variants with over expression of P-glycoprotein, MRP1, BCRP, Bcl-2, or Bcl-XL, suggesting that Shikonin can circumvent cancer drug resistance mediated by drug transporters or anti-apoptoticBcl-2 proteins [19].
Another necroptosis-induced natural product is Obatoclax (GX15-070), which is a small molecule bipyrrole compound that triggers necroptosis via formation of necrosome on autophagosomal membranes [20]. This complex assembly provide a connection between signaling pathways of necroptosis and Obatoclax-stimulated autophagy [20]. Obatoclax promote physical interaction between Atg5, a component of autophagosomal membranes with RIPK1 and RIPK3 (two key components of necroptotic signaling) [20]. Additionally, autophagy-dependent necroptosis was found to overcome resistance toward glucocorticoids in childhood acute lymphoblastic leukemia (ALL) [21].
Staurosporine triggers necroptosis in leukemia cells when caspase activation is inhibited [22]. This natural product is an inhibitor of protein kinases and the Staurosporine dependent necroptosis is blocked by Necrostatin-1 (RIP1 inhibitor) and necrosulfonamide (an inhibitor of MLKL) [22]. In contrast, the enzymatic role of PARP1 was found to be dispensable for staurosporine-induced necroptotic cell death [22].
BI2536 is another cancer specific necroptotic-trigger natural product. It is a small-molecule inhibitor of the mitotic kinase polo-like kinase 1 (Plk1). It initiates cell death by employing necroptosis in androgen-resistant prostate cancer cells [23]. This was concluded from experiments that showed: (1) Necrostatin-1 (an inhibitor of RIP1 kinase) attenuates cell death induced either by the Plk1 inhibitor BI2536 or by Plk1 silencing, (2) in cells where BI2536 induced cell death, there was no caspase activation, and (3) Using live cell imaging techniques, the morphological features of cell death was consistent with necroptotic cell death [23]. So, using BI2536 as an inhibitor of Plk1during mitotic progression results in mitotic catastrophe that ends with cell death by necroptosis.
FTY720 is a sphingolipid analog that mimics ceramide. It induces necroptotic cell death by causing changes in lipid signaling [64]. FTY720 binds to inhibitor 2 of PP2A (I2PP2A/SET) in the nucleus. I2PP2A is an on coprotein that inhibits PP2A (tumor suppressor enzyme protein phosphatase 2A) [24]. Upon binding of FTY720 to I2PP2A/SET, it reactivates PP2A and results in RIPK1-dependent necroptosis and suppression of lung cancer [24]. To confirm if the cell death was due to necroptosis, the authors used Necrostatin-1 to inhibit RIP1 as well as knockdown or genetic loss of RIP1 prevented the FTY720-indeced growth inhibition, thus gives strong conclusion that cell death was due to necroptosis. Experiments reconstituting wild type or mutant RIP1 constructs in RIP1 knockout MEFs under scored that RIK1 kinase activity was required for FTY720-induced necroptosis, since death-domain-deleted RIP1, but not the kinase-domaindeleted RIP1 restored FTY720-mediated necroptosis in MEFs lacking RIP1 [24].
In addition to previously mentioned compounds, there are some apoptotic-induced agents that have been shown to induce necroptosis under certain conditions. For example, at acidic extracellular pH, TRAIL (the death receptor ligand) was shown to induce necroptosis in colon and hepatocellular carcinoma cells [25]. RIPK1 or PARP1 pharmacological inhibitors as well as genetic blockage of RIPK1 or RIPK3 results in reduction of TRAILmediated necroptosis. Moreover, these interventions reduced intracellular PARP-dependent ATP depletion, indicating that both RIPK1&3 act upstream of PARP in this type of cell death [25]. In a mouse model of concanavalian A (Con A)-caused murine hepatitis (depend on TRAIL and Natural Killer (NK) T-cells for death of mouse hepatocytes), the liver injury was shown to be correlated positively with PARP1 activity. These effects was rescued by the inhibition of RIP1 or PARP1 [25].
Conclusion
In conclusion, necroptosis can be triggered in drug-resistant cancers that fail to induce apoptosis. Therefore, induction of necroptosis confers benefits in killing cancer cells and in improving immune responses to molecules released from dying cells even if these molecules can also promote neoplasia. Rhabdomyosarcoma exposed to Obatoclax results in autophagy that results, in turn, in activation of RIP3 and necroptotic cell death [26]. Of particular importance, in these cells caspase-8 didn’t inhibit RIP3 activation in autophagosome driven necroptotic process, unlike receptor mediated necrop¬tosis [26]. These data highlight that understanding the cross talk between necroptosis and other cell death types is a prerequisite for selecting optimal treatments customized to specific types of cancer and necroptosis associated diseases. Finally, a better understanding of the molecular events that regulate necroptotic cell death in different types of human cancers is expected to provide exiting novel opportunities in the coming years for therapeutic exploitation of cell death programs for the treatment of cancer.
References
- Fulda S, Gorman AM, Hori O, Samali A: Cellular stress responses: Cell survival and cell death. Int J Cell Biol 2010; 214074.
- Haanen C, Vermes I: Apoptosis: Programmed cell death in fetal development. Eur J Obstet Gynecol Reprod Biol. 1996; 64: 129 133.
- Opferman JT: Apoptosis in the development of the immune system. Cell Death Differ. 2008; 15: 234 242.
- Duval D, Trouillas M, Thibault C, Dembelé D, Diemunsch F, et al: Apoptosis and differentiation commitment: Novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2006; 13: 564 575.
- Giampietri C, Starace D, Petrungaro S, Filippini A and Ziparo E: Necroptosis: Molecular signalling and translational implications. Int J Cell Biol. 2014: 490275.
- Lu JV, Chen HC, Walsh CM. Necroptotic signaling in adap¬tive and innate immunity. Semin Cell Dev Biol. 2014: 35; 33 39.
- Fulda S. The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther. 2013; 14: 999 1004.
- Zhou W and Yuan J: Necroptosis in health and diseases. Semin Cell Dev Biol 35: 14 23, 2014.
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015; 517: 311-320.
- Moreno-Gonzalez G, Vandenabeele P, Krysko DV. Necroptosis. A novel cell death modality and its potential relevance for critical care medicine. Am J RespirCrit Care Med. 2016; 194: 415-428.
- Tummers B, Green DR. Caspase-8. Regulating life and death. Immunol Rev. 2017; 277: 76-89.
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015; 517: 311-320.
- Moreno-Gonzalez G, Vandenabeele P, Krysko DV. Necroptosis. A novel cell death modality and its potential relevance for critical care medicine. Am J RespirCrit Care Med. 2016; 194: 415-428.
- Grootjans S, VandenBerghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis. An overview. Cell Death Differ. 2017; 24: 1184-1195.
- Tummers B, Green DR. Caspase-8. Regulating life and death. Immunol Rev. 2017; 277: 76-89.
- Wegner KW, Saleh D, Degterev A. Complex Pathologic Roles of RIPK1 and RIPK3. Moving Beyond Necroptosis. Trends Pharmacol Sci. 2017; 38: 202-225.
- Dondelinger Y, Declercq W, Montessuit S, , Roelandt R, Goncalves A et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971- 981.
- Wang H, Sun L, Su L, Rizo J, Liu L et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014; 54: 133-146.
- Han W, Li L, Qiu S, Lu Q, Pan Q, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007; 6: 1641-1649.
- Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis bypromoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013; 20: 1161– 73.
- Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, etal. Induction of autophagy-dependent necroptosis is required for childhood acutelymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010; 120: 1310–1323.
- Dunai ZA, Imre G, Barna G, Korcsmaros T, Petak I, et al. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937cells. PLoS One. 2012; 7: e41945.
- Deeraksa A, Pan J, Sha Y, Liu XD, Eissa NT, et al. Plk1 is upregulated in and rogen-insensitive prostate cancer cells and its inhibition leads to necrop-tosis. Oncogene. 2013; 32: 2973–2983.
- Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2ARIPK1-dependent necroptosis. EMBOMol Med. 2013; 5: 105– 21.
- Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, LeMoigne-Muller G, et al. TRAIL induces necropto-sis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012; 19: 2003–2014.
- Basit F, Cristofanon S and Fulda S: Obatoclax (GX15 070) trig¬gers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013; 20: 1161 1173.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.