- Research Article
- |
- Open Access
Oncotype DX RS correlation with clinicopathologic risk factors and chemotherapy. Retrospective study in early stage ER positive breast cancer
- Farouq Salih;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Francois Calaud;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Kakil Rasul;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Mufid Elmistiri;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Nabil Elhadi;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Hafez Gazouani;
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar
- Salha Bujassoum
- Department of Medical Oncology, Breast Cancer Team, National Center for Cancer Care and Research -HMC - Qatar

| Received | : | Sep 26, 2018 |
| Accepted | : | Oct 25, 2018 |
| Published Online | : | Nov 02, 2018 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Salih F, Calaud F, Rasul K, Elmistiri M, Elhadi N, et al. Oncotype DX RS correlation with clinicopathologic risk factors and chemotherapy. Retrospective study in early stage ER positive breast cancer. Ann Breast Cancer. 2018; 1: 1005.
Abstract
Background: Oncotype DX risk score, a clinically validated test that estimates the recurrence and predicts the likelihood of benefit from adjuvant chemotherapy in early ER\PR positive, node-negative breast cancer, it is calculated based on characteristics of 21 genes that define the ER status, Her2 neu status, tumor proliferation, and tumor invasion. NCCN guidelines recommend adjuvant endocrine therapy for low RS (<18) and systemic adjuvant chemotherapy for high RS (>30), but no clear consensus about chemotherapy role in intermediate RS [18-30].
The aim of the study: Look for Oncotype Dx correlation, with clinicopathologic risk factors (age, tumor histology, tumor size, tumor grade, ER/PR status, tumor proliferation index) and chemotherapy. We did also evaluate how John Hopkins university recurrence score online tool can be utilized in filtering patient for Oncotype DX testing.
Methods: Retrospective records review of approximately 54 patients who had Oncotype DX test during 2012-2017 in National Cancer Center–Qatar.
Results: Of 54 patients studied 64.8% had low RS, 27.8% had intermediate RS, and 7.4% had high RS. Univariate analysis showed significant correlation with tumor grade (p<0.003), PR% status (cut-off 1%; p<0.016) and Ki67% (cut-off 20%; p<0.001). There was no significant correlation with patient age, tumor histology or tumor size. In multivariate analysis, only Ki67% predicted the Oncotype DX RS (p<0.028). JHU recurrence score had a moderate association with Oncotype DX RS at strength of agreement 0.524 (Cohen Kappa)
Adjuvant chemotherapy treatment correlated significantly with the Oncotype DX RS in both univariate analysis (p < 0.002) and multivariate analysis (p < 0.003)
Conclusion: Oncotype RS correlates significantly with the tumor grade, Ki67%, PR status, and chemotherapy treatment. JHU recurrence score has reasonable utility in filtering patient for Oncotype DX testing.
Keywords: Oncotype DX; Retrospective study; positive breast cancer; chemotherapy treatment
Introduction
Oncotype DX risk score, a clinically validated test that estimates the recurrence and predicts the likelihood of benefit from adjuvant chemotherapy in early node negative, hormonal receptors positive breast cancer treated with endocrine therapy [1]. It is calculated based on characteristics of 21 genes that define the ER status, Her2 neu status, tumor proliferation, and tumor invasion [1]. Node-negative breast cancer with low RS (<18, predicted 10 years recurrence 7%) gains little or no benefit from adjuvant chemotherapy while those with a high RS (>30, predicted 10 years recurrence 31% ) derive much benefit [2]. patients with intermediate Oncotype DX risk score (18-30, predicted 10 years recurrence 14%) [3], did not derive benefit from adding chemotherapy to the endocrine therapy as recently found by TAILORx trial [4], except some for patients 50 years of Age or younger with a recurrence score of 16 to 25.
The assay has been used in the adjuvant treatment planning of node-negative breast cancer and was incorporated into commonly accepted guidelines (NCCN, ASCO, ESMO, and St Gallen consensus).
At ESMO congress 2016, results were shared from two studies, the SEER (Surveillance, Epidemiology and End Results) program of the National Cancer Institute (NCI) [5] and the Clalit health services [6] showing the clinical utility of Oncotype DX RS in predicting breast cancer outcomes in patients with earlystage, node-positive disease. Oncologist started utilizing Oncotype DX RS for similar patient with node-positive breast cancer.
Shreds of evidence that show molecular tests have a role in individualizing therapy of early breast cancer are increasing [7]. Correlating clinical and pathological risk factors with Oncotype DX RS will be of benefit in identifying factors which are closely related and provide an overall estimate of a likely Oncotype DX risk category for particular patient before requesting the test.
The John Hopkins breast cancer recurrence estimator [8] an online tool that, gives an approximation of the Oncotype DX RS by entering the ER\PR percentage, Ki67% and grade of the tumor. It is intended to filter patient with early hormone receptorpositive breast cancer for Oncotype testing. Patients with low risk of recurrence identified by the estimator can be deferred Oncotype testing and treated accordingly with endocrine therapy, but how reliable is this?
We studied the correlation between clinical\pathological factors, Oncotype DX, and chemotherapy; we also evaluated how JHU recurrence score tool can be utilized in filtering patient for Oncotype DX testing.
Materials and methods
Patients
The study was performed in national cancer center–Qatar. The medical research committee approved the protocol with reference (MRC-01-18-128). Patients were eligible for the study if they were hormone receptors positive, her2 negative, early breast cancer diagnosed between 2012-2017 and had Oncotype DX test in the institutional database. 54 patients were identified retrospectively and included for the study. Clinicopathologic parameters including age, histologic type, tumor size, tumor grade, ER status, PR status, Ki-67 index, John Hopkins recurrence score, as well as ODxRS and chemotherapy treatment were obtained from the medical record for each patient.
Statistical Analysis
Statistical analysis using ordinal (Oncotype DX, JHU risk score) and nominal data (clinicopathologic factors). The correlation of Oncotype DX RS with clinicopathologic factors and chemotherapy was done using Pearson chi-square for univariate analysis and logistic regression for multivariate analysis.
Correlation of Oncotype DX RS with JHU recurrence score was done by using the Cohen Kappa correlation test and matching.
All tests were 2 tailed and with P-value ≤0.05 considered to be statistically significant.
Results
Oncotype DX RS utilization
Oncologist in our institute started requesting Oncotype DX test for the eligible patient after validation of the test through analysis of tumor bio-specimens from patient enrolled in the NSABP –B14 clinical trial 2011.
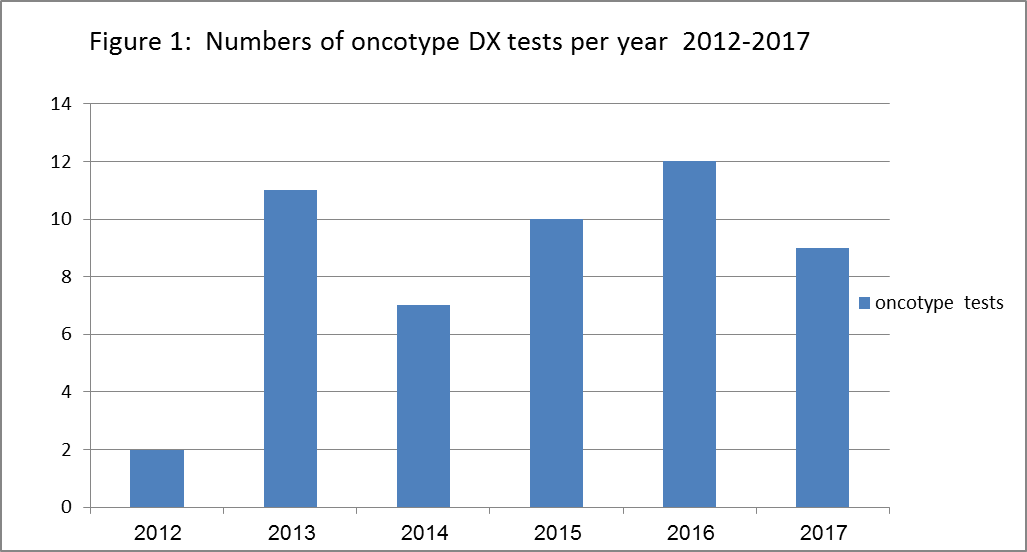
In 2012 our record showed that only 2 patients had the test, compared to 11 in 2013, 7 in 2014, 10 in 2015, 12 in 2016 and 9 in 2017 (Figure 1). There was almost constant utilization for his test since 2013. The average tests performed per year was 10. From mid-2017, Oncotype DX testing for node-positive her 2 negative early breast cancer was noted.
Patient and tumor description
In total, 54 patients were enrolled, (table 1) summarize the patient’s characteristics. The median age of studied patients was 49 years old (range 30-73 years) with 55.6% ≤ 50 years old. The major histology type was invasive ductal carcinoma reaching 90.7%. The median tumor size was 1.7 cm (range 0.6-4.5 cm). 57.4% (n=31) of the studied tumors were grade 2 by Nottingham grading system, 35.2% (n=19) were grade 1, and 7.4% (n=4) were grade 3. Overall, 51 (94.4%) patient were PR positive and only 3 (5.6%) were PR negative. Most of the patients 75.9% (41\54) had tumor with low proliferation index ki67% ≤20.
Oncotype DX correlation with clinicopathologic factors
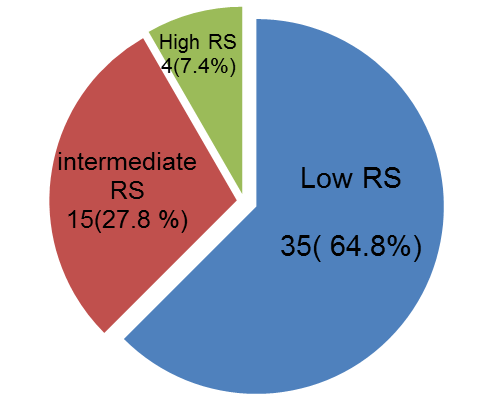
Of the 54 patients studied 35 (64.8%) patients had a low score, 15 (27.8%) patients had an intermediate score, and 4 (7.4%) had a high score (figure 2). Univariate analysis (Pearson chi-square) showed no significant correlation with patient’s age (P =0.68), tumor histology (p 0.99) or size (P =0.92). Three of the studied clinicopathologic risk factors including tumor grade (p<0.003), PR% status (cut-off 1%; p<0.016) and Ki67% (cut-off 20%; p<0.001) significantly correlated with Oncotype DX RS in univariate analysis.
Patients of age ≤50 years old were majority 60% (n 21/35) in the low-risk group of compared to 46.7% (n 7/15) in intermediate and 50% (n 2/4) in the high-risk group. Prevalence of patients who are ≤50 years old was approaching 39% in the lowrisk group compared to 16.7% in the remaining risk groups. The age did not show significant correlation with Oncotype DX as mentioned above.
The tumor size did not show a significant correlation to Oncotype DX. Tumor size ≤2 cm (T1) was the measure in 68.6% (n 24/35) of low risk tumors, 73.3% (n 11/15) of intermediate and 75% (n 3/4) of the high risk tumor, While tumor size > 2 cm (T2) reported in 31.4% (n 11/35) of low-risk tumors compared to 26.7% (n 4/15) of intermediate and 25% (n 1/4) of the high-risk tumors, while Tumor grade was one of the three variables that, showed significant correlation with Oncotype DX RS (p<0.003). 66% (n 33/50) of patients who had low-grade tumor (G1\G2) were reported in the low-risk group, representing 61% of the whole sample. On the other side, 30% (15/50) of patient classified as low-grade tumor (G1/G2) reported in intermediate and 4% (2/50 in high RS).
Low RS patients are more likely to express progesterone receptors 68.6% (LR 8, P < 0.017).
All progesterone receptor negative tumors were noticed to be of an intermediate score.
ki67% did differ significantly between Oncotype DX RS groups. 30 patients with Ki67 ≤ 20% had low-risk Oncotype DX, compared to 11 patients in the intermediate /high RS category
Despite the significant correlation of three pathologic factors (tumor grade, PR% status and Ki67%) with Oncotype DX RS in univariate analysis, only ki67% predicted the Oncotype DX RS in multivariate analysis, (OR 4.36 CI 1.17-16.23 P<0.028).
Oncotype DX RS association with JHU recurrence score
The John Hopkins breast cancer recurrence score is estimated from the online equation that uses the percentage of tumor cells that express ER and PR, ki67% and tumor grade [8]. The result is either low or undetermined risk of recurrence. In our cohort ( table 3), From the 35 patients reported with low Oncotype DX RS, JHU recurrence score predicted accurately the result of 28 patients (80%), all 7 (20%) remaining patients had intermediate RS, and none of them had high RS.
All the 4 (100%) patients with high Oncotype DX RS had undetermined JHU recurrence score.
when patients with intermediate\high Oncotype DX risk score are matched to either low or undetermined JHU score, the strength of agreement (Cohen Kappa) between Oncotype DX and JHU recurrence score was moderate at Kappa = 0.524 (95% confidence interval: 0.290 to 0.759).
Oncotype DX and clinicopathologic factors correlation with chemotherapy
Oncotype DX RS strongly correlated with chemotherapy in both univariate (p <0.002) and multivariate analysis (P< 0.003) (table 4A). Of the total 54 patients, 12 patients (22.2%) received adjuvant chemotherapy. Chemotherapy was given to 3/35 (8.6%) patient with low, 6\15 (40%) patient with intermediate and 3 of 4 patients (75%) with high Oncotype DX RS. 2 of the 3 patient in the low-risk Oncotype DX RS group had adjuvant chemotherapy because of tumor size. In the high-risk Oncotype DX RS group, 1 patient refused adjuvant chemotherapy because of personal preference. Having intermediate to high Oncotype DX RS demonstrated the highest predictive value for adjuvant chemotherapy treatment with an OR of 32 (95% CI, 2.4-411.4; P <0.008). Of the 42 patients who did not receive adjuvant chemotherapy, 76.2% belonged to low-risk group versus 23.8% categorized into intermediate/high groups. Overall, low-risk Oncotype DX RS patients were far less likely to receive adjuvant chemotherapy than those with high-risk Oncotype DX RS.
Patient age, tumor histology, Tumor size, tumor grade, PR status, and ki67%) show no correlation with adjuvant chemotherapy treatment in neither univariate nor multivariate analysis (table 4B).
Discussion
Oncotype DX RS had impact on the treatment of early nodenegative ER+ breast cancer, as prognostic test of 10 years metastases occurrence and predictive of response to treatment with tamoxifen versus tamoxifen plus chemotherapy; [10,11]. The importance of the test for node-positive ER+ cancer is evolving; however the evidence on analytical validity of Oncotype DX is partial, as there is no gold standard test with which the Oncotype DX can be compared. Our study results suggest correlation with tumor grade, PR score and Ki67%, as shown in some studies with similar objectives.
Ki67 testing was the only significant predictor of the Oncotype DX risk score when we did the multivariate analysis. This finding reduces the significance of contribution of tumor grade and the PR score to the Oncotype risk score and sub selects the Ki67% as the highest correlating factor with the Oncotype DX from the three factors. Despite this finding in multivariate analysis all three pathologic factors should be considered to identify those patients who require more careful evaluation of prognostic parameters and potentially molecular testing.
The JHU recurrence score did include the above significant pathologic factors, it had a moderate level of agreement, and could be useful when the result is undetermined, as for these patients Oncotype DX RS testing will be needed for treatment optimization. Patients who had low-risk JHU recurrence score might be spared the cost of the molecular test, giving that 80% end up with low and 20% will be an intermediate molecular score, however, both the oncologist and the patient might not feel comfortable to skip the more reliable molecular test. The JHU risk score might be reasonable option to use for patients with limited finance and will help in planning of their care.
Similar recurrence score tools to JHU are available for use. University of Tennessee health science center recurrence score [12] is an example of these estimation tool, but it was not included in this study, and it might be worthy finding its level of agreement with Oncotype DX risk score.
The molecular testing had effect on the decision of adjuvant chemotherapy in early breast cancer as an independent predictor of the likelihood benefit from chemotherapy currently for node negative and probably for node positive early breast cancer in the near future.
Is that enough? Probably not.
Combining the genomics of the tumor with other characteristics might give additional tools for more accurate prognosis that help the professionals in individualization and optimization of early breast cancer treatment. This point was explored by at least one study the Endopredict [13,14]. It was a retrospective analysis of 1702 postmenopausal ER+/HER2− breast cancer patients from two adjuvant phase III trials (ABCSG6, ABCSG8) treated for 5 years with endocrine therapy. The test incorporated genomics, tumor size and node status together into the EPclin score (low <3.3 high >3.3) that estimates the 5 and 10 years breast cancer recurrence (low < 10%, high >10%). The addition of the EP score to clinico-pathological parameters resulted in improvement of the prognostic performance of the test. Interestingly the Endopredict study did show that expression levels of proliferative and ER signaling genes contribute differentially to the early and late distant metastases. A high expression of genes–thought to contribute to cell cycle progression is significantly associated with higher rates of distant metastasis during the first 5 years but no longer shows a significant additional prognostic performance during the timespan thereafter. In contrast, genes associated with ER signaling were not significantly associated with early metastases but showed additional prognostic information in the second time. Personalized estimate of relapse risk after 5 years of endocrine treatment can improve patient selection for extended treatment with endocrine therapy The EPclin stratified 64% of patients at risk after 5 years into a low-risk subgroup with an absolute 1.8% of late DM at 10 years of follow-up.
Conclusion
Oncotype DX RS correlates significantly with the tumor grade, Ki67%, PR status, and chemotherapy treatment. The validity of the Oncotype DX test is partial and more knowledge of the characteristics and genomics, particularly genes with influence on proliferation and dissemination will help to produce tools that estimate the prognosis more accurately and predicts additional therapeutic benefits.
JHU recurrence score has reasonable utility in filtering patient for Oncotype DX.
The evidence of improvement in clinical outcome for patients treated based on Oncotype DX results still to be determined in the future, with prospective cohort studies and prolonged clinical follow up times.
Ethics Committee Approval
Ethics committee approval was received for this study. Reference (MRC-01-18-128).
Acknowledgments
The authors would like to thank the Medical research center, Medical oncology department, Breast cancer team, Department of Pharmacy, National Center for Cancer care and research, Hamad Medical Corporation, Qatar.
References
- Kim C, Baker J, Cronin M, Baehner FL, Walker MG, et al. A multigene assay to predict recurrence of tamoxifen-treated, nodenegative breast cancer. N Engl J Med. 2004; 351: 2817–2826.
- Albain K, Barlow W, Shak S, Horobagyi G, Livingston R, et al. Prognostic and Predictive Value of the 21-Gene Recurrence Score Assay in a Randomized Trial of Chemotherapy for Postmenopausal, Node-Positive, Estrogen Receptor-Positive Breast Cancer. Lancet Oncol. 2010; 11: 55–65.
- Ahn SG, Lee HM, Lee HW, Lee SA, Lee SR, et al. Prognostic discrimination using a 70-gene signature among patients with estrogen receptor-positive breast cancer and an intermediate 21- gene recurrence score. Int J Mol Sci. 2013;14: 23685–23699.
- Sparano JA. TAILORx: Trial Assigning Individualized Options for Treatment (Rx). Clin Breast Cancer N Engl J Med. 2018; 379:111- 21.
- Roberts MC, Miller DP, Shak S, Petkov VI. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Cancer Res Treat. Springer US; 2017; 163: 303–310.
- Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. npj Breast Cancer. 2017; 3: 32.
- Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016; 34: 1134–1150.
- Kim H, Umbricht CB, Illei PB, Cimino-Mathews A, Cho S, et al. Optimizing the Use of Gene Expression Profiling in Early-Stage Breast Cancer. J Clin Oncol. 2016; 34: 4390–4397.
- Tang G, Cuzick J, Costantino JP, Dowsett M, Forbes JF, et al. Risk of recurrence and chemotherapy benefit for patients with nodenegative, estrogen receptor-positive breast cancer: Recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol. 2011; 29: 4365–4372.
- Stemmer SM, Steiner M, Rizel S, Soussan-Gutman L, Ben-Baruch N, et al. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: Evidence from a large prospectively designed registry. npj Breast Cancer. 2017; 3: 33.
- Albanell J, González A, Ruiz-borrego M, Alba E, García-saenz JA, et al. Prospective trans GEICAM study of the impact of the 21- gene recurrence score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol. 2012; 23: 625–631.
- Orucevic A, Bell JL, McNabb AP, Heidel RE. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Research and Treatment. 2017; 163:51-61.
- Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, et al. The Endo Predict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br J Cancer. 2013; 109: 2959-2964.
- Peláez-García, Alberto, Yébenes L, Berjón A, Angulo A, Zamora P, et al. Comparison of risk classification between EndoPredict and Mamma Print in ER-positive/HER2-negative primary invasive breast cancer. PLOS one. 2017; 12: e0183452.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.