- Research Article
- |
- Open Access
Failure Patterns between HER2-Negative and HER2-Positive Luminal B Breast Cancer
- Noorie Choi;
- Department of Radiation Oncology, Seoul National University Bundang Hospital
- Department of Radiation Oncology, Veterans Health Service Medical Center
- Sea-Won Lee;
- Department of Radiation Oncology, Seoul National University Bundang Hospital
- Yujin Lim;
- Department of Radiation Oncology, Seoul National University Bundang Hospital
- Eunyoung Kang;
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea
- Eun-Kyu Kim;
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea
- Yu Jung Kim;
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea
- Jee Hyun Kim;
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea
- So Yeon Park;
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea
- In Ah Kim
- Department of Radiation Oncology, Seoul National University Bundang Hospital
- Breast Care Center, Seoul National University Bundang Hospital. Seongnam-si, Gyeonggi-do, South Korea

| Received | : | Apr 12, 2019 |
| Accepted | : | May 21, 2019 |
| Published Online | : | May 27, 2019 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Choi N, Lee SW, Lim Y, Kang E, Kim IA, et al. Failure patterns between her2-negative and her2- positive luminal B breast cancer after adjuvant post-operative radiotherapy. Ann Breast Cancer. 2019; 2(1): 1010.
Abstract
Majority of LBH+ patients undergo some form of systemic treatment and show relatively better clinical outcomes than LBH- patients. However, LBH- patients have poorer prognosis and may need more aggressive systemic approaches for improved treatment outcomes. Better differentiation of molecular subtypes is still a challenge and tumor protein p53 may be a potential marker for more refined risk stratification
Keywords: Breast neoplasms; Subtype; luminal; HER2; Adjuvant radiotherapy
Introduction
Breast cancer is a heterogeneous disease entity of various subtypes each with a distinct risk profile and natural history that entails an individualized treatment approach. Based on the widening knowledge behind its cancer biology, surrogate definitions of intrinsic molecular subtypes according to immunohistochemistry (IHC) and gene expression profiling were first introduced in the early 2000s and have since been established as a fundamental prognostic marker [1-4]. This intrinsic classification largely distinguishes breast cancer into basal-like, luminal, and human epidermal growth factor receptor 2 (HER2) overexpressing subtypes. Molecular subtyping has demonstrated prognostic significance for further subgrouping luminal breast cancer into A and B subtypes, where the latter shows increased prevalence of worse prognosis, earlier relapse, and higher levels of tumor proliferation. Significant progress has been achieved for improved objective risk profiling in the past decades and properly distinguishing the molecular subtype has become a crucial prerequisite for treatment planning [5-8]. The 2013 St. Gallen International Expert Consensus recommendation has suggested that cut-off values of IHC expression of Progesterone Receptor (PR) and Ki-67 set at 20% and 14%, respectively, allow for improved breakdown of subtypes (2). Though defining luminal A and HER2-enriched breast cancer is relatively explicit, reaching consensus on a refined classification within the luminal B subtype has been difficult due to its highly diverse clinical and molecular characteristics. Current standards stratify luminal B breast cancer into HER2-negative (LBH-) and HER2-positive (LBH+) subgroups, but still its definition criteria are vague and use broad conjunctions such as “or” and “any”. This study aims to compare clinical outcomes between the 2 subgroups of the luminal B subtypes.
Materials and Methods
Patients
From August 2003 to March 2012, 412 women diagnosed with either luminal B breast cancer underwent local surgical resection and Postoperative Radiotherapy (PORT). After excluding 4 patients who were diagnosed with ductal carcinoma in situ, 5 patients with distant metastasis at initial presentation, 3 patients with equivocal results for HER2 on both IHC and Fluorescence In Situ Hybridization (FISH), 3 patients who received treatment for a recurred disease, and 3 patients who were lost to follow-up, a total of 394 patients were analyzed.
Information relevant to patient demographics, clinicopathologic and treatment characteristics, and follow-up data on clinical outcomes were collected and reviewed. All patients were pathologically staged according to the 7th edition of the TNM staging system provided by the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Approval for retrospective review of medical records was obtained from the Institutional Review Board.
Surgical resection included Breast-Conserving Surgery (BCS) and Modified Radical Mastectomy (MRM). Chemotherapy was prescribed accordingly by the medical oncologist. The most common regimen for chemotherapy was Adriamycin and Cyclophosphamide. Trastuzumab was prescribed for patients with HER2 overexpression.
All patients were irradiated with a median total dose of 59.4 Gy (range 50-66) to the whole breast or chest wall. The prescription of a tumor bed boost and the extent of including regional lymph nodes were decided upon at the discretion of the radiation oncologist.
Molecular subtype definitions
Molecular subtypes were assessed based on IHC expression of Estrogen Receptor (ER), PR, HER2, and Ki-67. Cut-line values for PR and Ki-67 were set to 20% and 14%, respectively [9, 10]. Patients were defined as either LBH- or LBH+ according to the 2013 St. Gallen International Expert Consensus recommendation (2). Definitions are described in Table 1. IHC scores of 2+ for HER2 protein expression were presumed as negative results until verified by a reflex test using a dual-signal FISH assay to evaluate for HER2 gene amplification. HER2 was considered positive if IHC was scored 3+ (protein overexpression) or if FISH showed a HER2/CEP17 ratio > 2.2 or HER2 gene copy number > 6.0 (gene amplification) [11]. In cases with discordance between HER2 testing methods, FISH assay results w interpretationere adopted for conclusive. Patients with luminal-type breast cancer and equivocal HER2 results on IHC and/or FISH were additionally categorized as luminal B HER2-equivocal if all featuresERpositive of luminal A breast cancer, excluding HER2, were absent (, PR ≥ 20%, and Ki-67 < 14%).
Definition of recurrence
Local recurrence was defined as Ipsilateral Breast Tumor Recurrence (IBTR) if the ipsilateral breast or chest wall, including surgical scars, was involved. Regional Recurrence (RR) was defined as tumor recurrence of regional lymph nodes in the ipsilateral axillary levels I, II, and III, internal mammary, infraclavicular, and supraclavicular areas. Tumor recurrence in the contralateral breast or chest wall, distant lymph nodes, or any other site was considered as Distant Metastasis (DM)
Statistical analysis
Clinicopathologic characteristics between LBH- and LBH+ breast cancers were compared using the independent samples t-test for continuous variables and chi-squared test for categorical variables. Primary endpoints for analysis were Local Control (LC), RR-free survival (RRFS), DM-free survival (DMFS), diseasefree survival (DFS), and overall survival (OS) rates. Time intervals were measured from the base of follow-up, defined as the date when initial treatment was initialized, to the occurrence of events: IBTR, RR, DM, or death. Survival rates were calculated and compared using the Kaplan-Meier method and log-rank test. IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY) was used for all statistical analyses. Statistical significance was defined as p < 0.05 with a two-tail approach.
Results
Clinicopathologic characteristics
A total of 394 breast cancer patients with stage I-III breast cancer were analyzed, including 258 (65.5) LBH- patients and 136 (34.5%) LBH+ patients. The median age of patients at diagnosis was 49 years (range 24-81) and the median follow-up duration was 6.3 years (range 1.1-13.0). Clinicopathologic characteristics, summarized in Table 2, demonstrated statistically significant differences in age at diagnosis, PR expression, expression of p53, and histologic grade. Higher proportions of patients were diagnosed at younger ages in the LBH+ subgroup. Histologic grade I tumors were more common in the LBH- subgroup, whereas grade II tumors were more common in the LBH+ subgroup. Majority of patients were diagnosed with invasive ductal carcinoma. Other histologic types included mucinous carcinoma, invasive micropapillary carcinoma, and pleomorphic lobular carcinoma. A total of 104 patients received neoadjuvant chemotherapy, of which 8 patients demonstrated post-treatment transformation of molecular subtype: from LBH- to luminal A in 4 patients and from LBH+ to HER2-enriched in 4 patients.
Surgical or hormonal therapy approaches were not significantly different between the 2 subgroups within the luminal B subtype. In relation to systemic chemotherapy, the LBH- subgroup had a higher propensity for omission (p=0.013). This difference was statistically significant for patients with stage I tumors (p < 0.001), but not for stage II-III tumors (p=0.474). Overall, 25 (6.3%) patients did not receive hormonal therapy, demonstrating no difference between LBH- and LBH+ subtypes.
Patterns of failure
When the patterns of failure were compared between the LBH- and LBH+ subgroups, any breast cancer recurrence occurred in 34 (13.2%) and 16 (11.8%) patients, LRR occurred in 14 (5.4%) and 5 (3.7%) patients, DM occurred in 29 (11.2%) and 13 (9.6%) patients, and death due to breast cancer was seen in 14 (5.4%) and 3 (2.2%) patients, respectively.
Patients diagnosed with LBH- breast cancer demonstrated a higher tendency of recurrence in multiple sites. Simultaneous involvement of multiple sites at the time of diagnosis of metastatic breast tumor recurrence was observed in 16 patients (47.1%) of the LBH- subgroup and in 4 patients (25.0%) of the LBH+ subgroup (p=0.137). Differences were not significant, but a higher proportion of lymphatic recurrence was observed in the LBH- subgroup. Though analysis of treatment outcomes did not demonstrate statistical significance, a generally worse pattern of prognosis was seen in the LBH- subgroup (Table 3). On multivariable Cox regression analysis, N stage in LBH- and N stage and histologic grade in LBH+ were identified as independent prognostic factors for relapse within 5 years. Among patients with positive p53 protein expression, the LBH- subtype demonstrated significantly higher rates of distant metastasis at 13.8% versus 2.0% in the LBH+ subtype (p=0.041) and especially higher rates of lung metastasis (9.2% versus 0%, respectively, p=0.034).
Survival outcomes
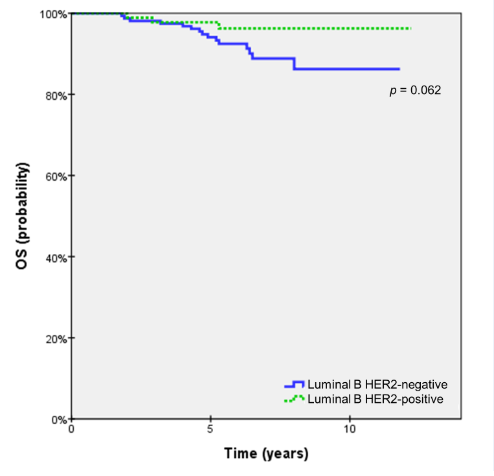
Survival rates were compared at 5- and 10-years of followup (Table 4). Though not significant, the LBH- subgroup demonstrated to have worse outcomes for all survival rates at 5-years. At 10-years, however, survival rates were either similar or better in the case of locoregional and distant failure. The OS rate was the only survival end-point that showed a trend toward statistical significance (p=0.062). The difference of OS rates was minimal at 5-years, 97.3% and 99.3% for LBH- and LBH+, respectively, but a growing gap between the subgroups was observed as OS rates fell to 87.9% and 97.0% at 10-years (Figure 1). Among patients that had p53 positivity, LBH- and LBH+ showed significantly different DMFS of 87.1% versus 98.0%, respectively (p=0.030), whereas no statistically significant difference was found in the p53-negative subgroup (Table 5).
Discussion
Luminal B breast cancers are a heterogeneous group among all molecular subtypes in terms of not only molecular expressions and clinical behavior, but also regarding patterns of care as well. Results of our study demonstrate that a significantly higher proportion of patients are being omitted from chemotherapy in the LBH- subgroup, whereas most patients of the LBH+ subgroup undergo some form of systemic therapy—either cytotoxic and/or immunologic.
One of the major rationales in distinguishing luminal breast cancers into A and B subtypes is to identify patients with comparatively better prognosis for whom chemotherapy may not be needed. Because luminal B breast cancers generally show worse prognosis, earlier relapse, and higher levels of tumor proliferation, more aggressive treatment is often needed. Luminal B breast cancers are not only highly diverse in terms of clinical and biomolecular behavior, but treatment patterns as well.
Effort to more precisely distinguish breast cancer into distinct molecular entities is still an ongoing challenge, especially for the luminal B subtype. Analyses of our data demonstrate that LBH- and LBH+ subtypes show differences in patterns of treatment outcomes. Though not statistically significant, patients with LBH- subtype of breast cancer had higher proportions of IBTR and RR. LBH- also demonstrated worse OS with a trend toward statistical significance at 10 years. Currently there is no widely accepted consensus on the indication for chemotherapy in patients of LBH- subtypes, thus patterns of care are highly heterogeneous. In general, patients with LBH- subtype had significantly higher rates of systemic treatment omission in stage I tumors, whereas LBH+ patients were more likely to receive either chemotherapy or targeted immunotherapy.
Several other biomarkers such as p53, epidermal growth factor receptor (EGFR), p16, and androgen receptor have been studied for its significance as molecular distinguishers. Positivity for p53 mutation has shown association with poorer treatment outcomes. Analysis of p53-positive luminal B breast cancer patients in our series showed that LBH- patients had significantly higher risk of DM than LBH+ patients. DMFS was significantly worse among p53-positive LBH- patients, whereas no difference between p53-negative LBH- and LBH+ patients were seen, possibly suggesting that the overexpression of the p53 protein may play a role in treatment outcomes. In a study of 294 patients by Overgaard et al., TP53 gene mutation showed to be a significantly strong predictor of OS and DFS in both node-negative and node-positive patients [12], while cells with wild type p53 have shown to be more radiosensitive [13]. Treatment outcomes are also influenced by expression levels of ER subtypes α (ERα) and β (ERβ) [14]. Among ductal breast cancers, high ERα levels were expressed for highly proliferative disease and low ERβ levels were expressed for early stage disease [15]. Studies have shown that ERα directly binds to p53 and inactivates tumor suppression. Ionizing radiation disrupts this interaction and induces p53 phosphorylation, which leads to cell cycle arrest or apoptosis due to disruption of the MDM2-p53 pathway [16- 18]. In context, hormonal therapy with ERα-antagonists and/or ERβ-agonists for estrogen-responsive breast cancer has shown to interact with ionizing radiation, improving LC and overall patient survival [19].
The role of radiation for inactivating ERα-p53 interactions and restoring function p53 has been suggested to be contributed by interactions of p53 with other regulatory proteins as well. Coexistence of p53 and HER2 overexpression demonstrated to have better prognosis than other combinations of protein expression in a study by Rosen et al [20]. This study analyzed 440 node-negative patients and reported that patients with both p53 and HER2 positivity showed best prognosis, while p53-negative HER2-positive patients had the poorest prognosis. This was most significant for recurrence-free survival and OS in T1N0M0 subgroup of patients. However, other studies have demonstrated that the expression of p53 with HER2 positivity had poorer prognosis [21-24]. Such inconsistency of results on the effect of p53 and HER2 coexistence may be due to the inability of IHC to detect the complete loss of p53 [25]. Furthermore, p53 overexpression on IHC does not always represent p53 gene mutations [26]. Though our results showed a possible correlation between p53 positivity and worse DMFS in LBH- patients, interpretation should be made carefully and needs further studies for clinical support.
Limitations of this study include possible bias due to its retrospective nature, a relatively small study population, and heterogeneous treatment patterns between patients of similar molecular subtypes. Beside the overexpression of p53, other biomarkers including EGFR and cyclooxygenase-2 were also analyzed, but due to a limited number of available data no statistical significance was found. Though our data was limited, additional information on ERα and ERβ expression levels and its relation in context to hormonal therapy may provide valuable information in better classifying luminal B breast cancers. Another limitation is that p53 assessment was solely based on results of IHC staining where expression positivity on IHC may not necessarily correlate with actual gene mutation. Further analysis will be needed to evaluate the clinical significance of p53 protein overexpression on IHC and assess the correlation between results of IHC and gene profile analyses.
Conclusion
Majority of LBH+ patients undergo some form of systemic treatment and show relatively better clinical outcomes than LBH- patients. LBH- patients have poorer prognosis and may need more aggressive therapeutic approaches.
Clinical practice points
Patients with luminal B HER2-negative breast cancer are more likely to be omitted from systemic chemotherapy
Overall survival rates are demonstrated to be lower in luminal B HER2-negative compared to HER2-positive breast cancer
Acknowledgments
This work was supported by a grant from the Ministry of Science and Information & Communication Technology (NRF#2017R1A2B4002710 & #2017M2A2A7A01018438) and SNUBH Research Fund (#14-2018-003) to In Ah Kim.
Ethical approval
This study was approved by the Institutional Review Board (B-1505/298-116) and was exempted from the requirement to obtain written informed consent from patients due to its retrospective design.
References
- Bediaga NG, Beristain E, Calvo B, Viguri MA, Gutierrez-Corres B, et al. Luminal B breast cancer subtype displays a dicotomic epigenetic pattern. Springerplus. 2016; 5: 623.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24: 2206-2223.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24: 2206-2223.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000; 406: 747-752.
- Cirqueira MB, Moreira MA, Soares LR, Cysneiros MA, Vilela MH, Freitas-Junior R. Effect of Ki-67 on immunohistochemical classification of luminal A to luminal B subtypes of breast carcinoma. Breast J. 2015; 21: 465-472.
- Ahn HJ, Jung SJ, Kim TH, Oh MK, Yoon HK. Differences in clinical outcomes between luminal A and B type breast cancers according to the St. Gallen Consensus 2013. J Breast Cancer. 2015; 18: 149-159.
- García Fernández A, Chabrera C, García Font M, Fraile M, Lain JM, Gónzalez S, et al. Differential patterns of recurrence and specific survival between luminal A and luminal B breast cancer according to recent changes in the 2013 St. Gallen immunohistochemical classification. Clin Transl Oncol. 2015; 17: 238-246.
- Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, et al. A revised clinico-pathological surrogate definition of luminal A intrinsic breast cancer subtype. Breast Cancer Res. 2014; 16: R65.
- Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013; 31: 203-209.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101: 736-750.
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31: 3997-4013.
- Overgaard J, Yilmaz M, Guldberg P, Hansen LL, Alsner J. TP53 mutation is an independent prognostic marker for poor outcome in both node-negative and node-positive breast cancer. Acta Oncol. 2000; 39: 327-333.
- Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006; 13: 293-325.
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014; 90: 13-29.
- Huang B, Omoto Y, Iwase H, Yamashita H, Toyama T, et al. Differential expression of estrogen receptor alpha, beta1, and beta2 in lobular and ductal breast cancer. Proc Natl Acad Sci USA. 2014; 111: 1933-8.
- Liu W, Ip MM, Podgorsak MB, Das GM. Disruption of estrogen receptor alpha-p53 interaction in breast tumors: a novel mechanism underlying the anti-tumor effect of radiation therapy. Breast Cancer Res Treat. 2009; 115: 43-50.
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nature Reviews Cancer. 2003; 3: 117-29.
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997; 91: 325-34.
- Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004; 96: 115-21.
- Rosen PP, Lesser ML, Arroyo CD, Cranor M, Borgen P, Norton L. p53 in node-negative breast carcinoma: An immunohistochemical study of epidemiologic risk factors, histologic features, and prognosis. J Clin Oncol. 1995; 13: 821-830.
- Yamashita H, Nishio M, Toyama T, Sugiura H, Zhang Z, et al. Coexistence of HER2 over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer. Breast Cancer Res. 2004; 6: R24-R30.
- Beenken SW, Grizzle WE, Crowe DR, Conner MG, Weiss HL, et al. Molecular biomarkers for breast cancer prognosis: Coexpression of c-erbB-2 and p53. Ann Surg. 2001; 233: 630-638.
- Sjögren S, Inganäs M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998; 16: 462-469.
- Isola J, Visakorpi T, Holli K, Kallioniemi OP. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992; 84: 1109-1114.
- Chang F, Syrjänen S, Syrjänen K. Implications of the p53 tumorsuppressor gene in clinical oncology. J Clin Oncol. 1995; 13:1009-1022.
- Wynford-Thomas D. p53 in tumour pathology: Can we trust immunocytochemistry? J Pathol. 1992; 166: 329-330.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.