- Review Article
- |
- Open Access
Control of breast cancer using metal-containing polymers based on cell line results
- Michael R Roner;
- Department of Biology, University of Texas Arlington, USA
- Charles E Carraher;
- Department of Chemistry and Biochemistry, Florida Atlantic University, USA
- Kimberly Shahi;
- Department of Biology, University of Texas Arlington, USA
- Alisha Moric-Johnson;
- Department of Biology, University of Texas Arlington, USA
- Lindsey Miller;
- Department of Biology, University of Texas Arlington, USA
- Paul Slawek;
- Department of Chemistry and Biochemistry, Florida Atlantic University, USA
- Francesca Mosca
- Department of Chemistry and Biochemistry, Florida Atlantic University, USA

| Received | : | Apr 23, 2018 |
| Accepted | : | Aug 01, 2018 |
| Published Online | : | Aug 08, 2018 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Roner MR, Carraher CE, Shahi K, Moric-Johnson A, Miller L, et al. Control of breast cancer using metal-containing polymers based on cell line results. Ann Breast Cancer. 2018; 1: 1003.
Abstract
Metal-containing polymers have shown good activity against MCF-7 and MDA-MB-231 human breast cancer cells. These cell lines represent about one half of the known human breast cancers. MDA-MB-231 (strain number 7233) cells are estrogen-independent; estrogen receptor negative while the MCF-7 (strain line 7259) cells are estrogen receptor (ER+) positive and the combination are used as a matched pair. Polyethers that contained the O-phenylene structural unit showed good inhibition to the non-estrogen sensitive cell line, MDA-MB-231, but were less effective towards the MCF-7 cells, a well-characterized estrogen receptor positive control cell line. Using camphoric acid as the model Lewis base, the most active polymers were derived from zirconocene and hafnocene-containing polymers and the Group 15 polymers were the least active. Organotin polymers showed intermediate ability to inhibit breast cancer cell growth.
Overall Objective
We have produced over 75 new families of metal-containing polymers for various purposes. These include the following metals: sodium and potassium as salts; organometallics containing titanium, zirconium, hafnium, vanadium, chromium, iron, cobalt, nickel, rubidium, platinum, palladium, silicon, germanium, tin, lead, phosphorus, antimony, bismuth, arsenic, uranium, etc. Some of these efforts have been reviewed [1-6]. The focus in the current paper is on Group 4 metallocene, Group 15 and organotin polymers and their ability to control breast cancer.
In this paper we introduce cell line data related to the use of metal-containing polymers with reference to controlling the growth of two human breast cancer cell lines. Based on the cell line results the ability to control breast cancer growth is evaluated relative to the nature of the metal.
Overall, the synthesis of the metal-containing polymers is based on the Lewis acid/base concept where an electron deficient Lewis acid is the metal-containing unit and the electron donating Lewis base is the non-metal containing unit.
Polymer Advantages
The use of polymeric drugs is wide spread including their use to inhibit cancer growth [7-19]. Some advantages in comparison to small, monomeric drugs are briefly described as follows.
First, because of their large size, they can be designed with particular components incorporated to impart desired characteristics including drug release or retention and if released, how fast it is to be released; polymer solubility, preferred location for activity, etc. This fine-tuning includes chain length, polarity, monomer characteristics, crosslinking, and preferred duration of activity. Second, the polymer chain can be designed to deliver the “drug” as part of the entire chain that enters the cell by pinocytosis [20,21] being active in the polymer form or sufficiently unstable as to release the drug in some sustained release mode [22-24]. Our polymers act against the cancer cells in tact rather than through some decomposition product. Third, when characteristics of solid cancers are listed, one that is often omitted, but that is important, is the difference in characteristic between healthy and cancer cell walls. Cell walls of cancer cells, compared to normal cells, are more ragged allowing polymer chains to become “hooked” resulting in a longer contact time and enhanced activity against these cancer cells in comparison to smoother healthy cells. Fourth, polymers are filtered out by the kidneys more slowly than small compounds [25] decreasing kidney damage and allowing for prolonged retention. Fifth, because of its large size the polymer can be designed to provide more bonding sites to cellular targets increasing their effectiveness. Sixth, polymers can be designed to contain several different anti-cancer agents. Thus, cisplatin and one of the polymers focused on in this paper can be incorporated in the same polymer chain offering different mechanisms for cancer inhibition. Seventh, polymers may be active against cancer cells that have developed resistance. This resistance is believed to be partially due to the cell being alerted through the prior presence of small molecule chemoagents. Special “housekeeping” proteins are alerted to the invasion of the original anticancer agents and are then prepared to do “warfare” when other invaders arrive [26]. Some of our polymers have been effective at inhibition of cell lines that have become resistant [6,27] possibility because of their polymeric nature compared to the smaller size of the typical anticancer agents.
Finally, as noted before, cancer cells are both irregular and leaky compared to normal cells with the potential of polymers occupying the interstitial space due to the leaky vasculature and limited lymphatic drainage typical of cancer cells [28]. This effect is called the enhanced permeability and retention, EPR, effect [17-19,28]. This listing is not exhaustive but highlights the advantages of polymers in the fight against cancer.
Synthesis
The polymers described here are synthesized employing commercially available reactants and a system that is employed in the industrial synthesis of polymers including aramid fibers and polycarbonates [29,30]. This allows for a somewhat straight forward scale-up from the milligram to ton amounts. As in almost all cases, while the process for scale-up is straight forward, the particulars are typically not.
The polymerization technique employed in the synthesis of the polymers is the interfacial polymerization discovered by Paul Morgan and coworkers at DuPont to synthesize the aromatic fibers referred to as aramid fibers [31]. This system has been modified by Carraher to include a variety of approaches [32- 34]. Here, the typical aqueous interfacial system is employed. A typical reaction system is described in the experimental section. Briefly, the Lewis base is dissolved in water. The Lewis acid is likewise dissolved in an organic solvent such as heptane that is immiscible with water. The two phases are added to a blender stirring at a rate of about 18,000 rpm. For comparison, this is over double the rotation rate of most model airplane motors. After less than one minute, generally almost immediately, polymer is formed. The reason for the rapidly of the reaction is the use of what is referred to as highly reactive reactants where the activation energy is in the general realm of 20 Kcal/mole or 80 Kjoule/mole.
Each of the polymers synthesized by us has undergone extensive chemical structural analysis including chain length, nuclear magnetic resonance spectroscopy, infrared spectral analysis and high resolution electron impact positive ion matrix assisted laser desorption time of flight mass spectroscopy (MALDI MS).
Experimental Synthesis
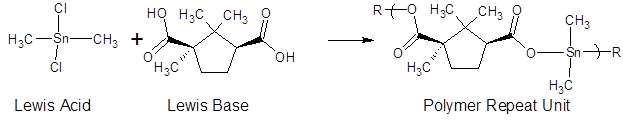
Reactions were carried out using the interfacial polycondensation technique. Following is a brief description for the reaction between typical reactants here camphoric acid as the Lewis base, and an organotin dichloride as the Lewis acid. An aqueous solution (30ml) containing the camphoric acid (0.00300mol) and sodium hydroxide ( 0.0060mol) is transferred to a one quart Kimax emulsifying jar fitted on top of a Waring Blender (model 1120; no load speed of about 18,000 rpm; reactions were carried out at room temperature about 25o C). Because the diacid form of camphoric acid is not a strong nucleophile, base is added converting camphoric acid to its salt which is a reasonably strong nucleophile. Stirring was begun and a heptane solution (30ml) containing the organotin dihalide (0.00300mol) was rapidly added (about 3-4 seconds) through a hole in the jar lid using a powder funnel. The resulting solution was blended for 15 seconds. The precipitate was recovered using vacuum filtration and washed several times with deionized water and heptane to remove unreacted materials and unwanted by-products. The solid was washed onto a glass petri dish and allowed to dry at room temperature.
Figure 1 contains the description for the reaction described in the experimental section.
Tumors Tested
The cell lines MDA-MB-231 and MCF-7 both originated from human breast cancer pleural effusions. Both are adenocarcinomas. The MCF-7 cell line is very sensitive to estradiol. The MCF-7 line retains several characteristics of differentiated mammary epithelium including ability to process estradiol via cytoplasmic estrogen receptors. The cell lines MCF-7 and MDA-MB-231, plus a third T-47D, account for more than two-thirds of all reported studies utilizing breast cancer cell lines [35]. The MDA-MB-231 cells form poorly differentiated adenocarcinomas (grade III) in ALS treated BALB/c mice and nude mice. Estradiol responsive tumors are produced in athymic mice using MCF-7 cells. The MCF-7 cells also express the WNT7B oncogene. The MDA-MB231 cells are a highly rearranged human cell line of female origin containing 59 to 66 chromosomes per metaphase spread. Structural abnormalities include rearrangements to over 60% of the 22 different autosomal chromosomes and to the X chromosome (ATCC® HTB-26™).
The MCF-7 cells are a highly rearranged human cell line of female origin containing 74 to 89 chromosomes per metaphase spread. Structural abnormalities include rearrangements to chromosomes X, 1, 2, 3, 5, 6, 7, 8, 9, 12, 13, 15, 18, 19, 20, and 22 (ATCC® HTB-22™).
Tumor Testing Experimental
The toxicity of each test compound was evaluated as follows. Following a 24 h incubation period, the test compounds were added at concentrations ranging to 60 microgram/mL and allowed to incubate at 37°C with 5% CO2 for 72 h. Following incubation, Cell Titer-Blue reagent (Promega Corporation) was added (20 uL/well) and incubated for 2 h. Fluorescence was determined at 530/590 nm and converted to % cell viability versus control cells.
All cytotoxicity values are calculated against a base-line value for each line that was generated from “mock-treatment” of the normal and tumor cell lines with media supplemented with all diluents used to prepare the chemotherapeutic compounds. For example, if the compounds were dissolved in Dimethyl Sulfoxide (DMSO) and serial dilutions prepared in MEM (minimum essential medium) to treat the cells, then the mock-treated cells were “treated” with the same serial dilutions of DMSO without added chemotherapeutic compound. This was done to ensure that any cytotoxicity observed was due to the activity of the compound and not the diluents. The mock-treatment never resulted in a loss of cell viability of more than one percent, demonstrating that the activity observed was not due to cytotoxicity of any of the diluents used, but was due to activity of the tested compounds.
Once inhibition begun, the slope of the concentration versus inhibition is steep and continues to total inhibition.
Diagnostic Tools
The two most widely accepted approaches for evaluating tumor cancer inhibition data are employed by us. The first involves the concentration required to inhibit cell growth some amount, generally 50%. This is referred to by a variety of names such effective concentration, EC, and for 50% inhibition this is described as EC50. This is a direct measure of the concentration of compound that inhibits cell growth. The second measure is again referred to by a variety of names such as chemotherapeutic index, CI. For measurements for 50% inhibition, this is the ratio for the concentration of drug needed to inhibit the growth of a standard 50%, here the WI-38 human healthy normal lung embryonic fibroblast cell, divided by the concentration of compound that inhibits the growth of that particular cell 50%. The values for the CI50 are taken directly from the EC50 measurements. The use of WI-38 cells is the most widely employed standard. These values are given in Tables 1 through 4 for selected studies.
Low EC50 values are preferred for the tested compounds compared to the standard WI-38 standard values. Further, high CI50 values are preferred since they show a great difference between the concentrations of tested compound to inhibit cancer growth compared to the concentration needed to inhibit the human healthy standard.
Structural Considerations
We have observed that Lewis bases that contain certain structural units have markedly different abilities to inhibit the two breast cancer cell lines. This difference is generally found when the Lewis base has structural units similar to drugs often employed to treat breast cancer, namely antiandrogens. Following demonstrates this for some of the polymer structures studied by us.
Initially our focus is on diethylstilbestrol, DES. DES is a synthetic estrogen that mimics estrogen. It is one of a group of synthetic estrogens used in the treatment of certain breast cancers. It is sold under of different names including cyren A, distilbene, Apstil and stilbetin. It was initially employed to prevent miscarriages or premature deliveries beginning in 1938. It was a horrible choice since it was later linked to a rare vaginal cancer found in female offspring. It is in fact a teratogen whose use may result in malformation of an embryo or fetus.
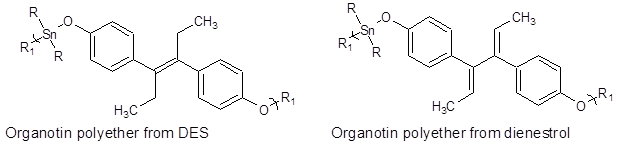
DES has been employed in the treatment of breast and prostate cancer but its use has been fortunately limited due to its poor solubility and a wide variety of toxicities. Its use in hormonal therapy of metastatic prostrate has been described (Figure 2).
Figure 2: Structures for the repeat unit for organotin polyethers formed from DES, left, and dienestrol, right.
The second hormone used for comparison is dienestrol, one of the most widely employed sex hormones. In literature it is often confused with DES because of its similar structure but it is a different chemical with its own unique chemistry and biological activities. It is sold under a variety of trade names including Lipamone, Retalon-Oral, and Farmacyrol. It is employed in hormone therapy mainly estrogen replacement therapy.
Table 1 contains results for polymers made from each of these hormones [36,37]. In all cases, the ability to inhibit the MDA cell line is markedly greater for the two polymers compared to the MCF cell line. For the polymers the ratio of MCF/ MDA ranges from 1.5 to 12 for DES and for dienestrol from 2.3 to 82. This difference is also shown for the monomers DES and dienestrol.
Table 1: GI50 concentrations (micrograms/mL) for organotin polyethers derived from diethylstilbestrol (DES) and dienestrol for MDA and MCF-7 cell lines [36,37].
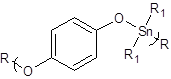
We have studied numerous polymers containing the C-O linkage that did not show this difference in ability to inhibit the two cell lines. The structural difference is that these two polymers contain the O-phenylene grouping whereas most of the other polyethers do not. This difference is also found for organotin polyethers derived from a wide variety of products derived from reaction with hydroquinone and hydroquinone derivatives (Figure 3). These polymers also contain the O-phenylene linkage.
Figure 3: Repeat unit for the product of hydroquinone and organotin dichloride where R represents simple chain extension and R1 various alkyl and aryl groups.
These results are consistent with a preferential inhibition of the MDA non-estrogen cancer cell line by these certain polymers. DES and dienestrol are more effective against Estrogen Receptor positive (ER+) tumors such as the MCF-7 cell line. It is possible that some of the polymer from the hormone drugs and hydroquinone derivatives is bound to the estrogen receptors in the MCF-7 cell line rendering it unavailable for action within the cell. Physicians treating breast cancer should be aware of this possibility in selecting treatments.
Relative Effect of Metal
One of the major reasons for our current research is to ascertain if there is a structural relationship relative to the ability to inhibit breast cancer growth. This section presents studies of related compounds differing only in the nature of the metal present. Here we are using a variety of metal-containing polymers based on reaction with camphoric acid. (We have observed that the chain length, polymer molecular weight, has little effect on the ability to inhibit cancer growth.) This combination represents typical data and will be employed to describe trends.
Table 2: contains EC50 and CI50 values for the reactants and polymers for the formation of the organotin polyesters formed from reaction with camphoric acid. The overall reaction is given in Figure 1.
Table 2: EC50 Concentrations (micrograms/mL) and CI50 values for the tested compounds [38]. Values given in ( ) are standard deviations for each set of measurements. The EC50 values are the concentrations, in micrograms of sample per milliliter of solution, where 50% of the particular cell line cited at the top of each column, are inhibited.
Organotin polymers typically give EC50 values lower than the organotin monomers with some in the nanogram/mL range. While the CI50 values for the polymers are generally greater than those for the organotin monomers, they are not high. As is common for other studies, the dibutyltin polymer gives decent results. This is advantageous because dibutyltin dichloride is the least expensive of the organotin halides and of any of the organometallic-reactants employed in this report. It is available in gram to ton amounts. Because of its widespread commercial use, more is known about it than any of the other organotin monomers [38]. It is the least toxic to humans of the organotin monomers [39]. Finally, in nature it degrades to simple tin oxide offering a low toxic form of degradation product [39].
It is important to note there is not general agreement between researchers whether EC or CI values are the most important indicators for future clinical testing.
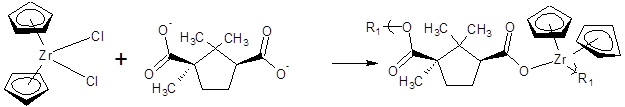
Table 3 presents EC50 and CI50 values for the monomers and products of camphoric acid and Group 4 metallocenes (Figure 4).
Table 3: EC50 Concentrations (micrograms/mL) and CI50 values for the monomers and polymers formed from reaction of camphoric acid and Group 4 metallocene dichlorides. Values given in ( ) are standard deviations for each set of measurements [40].
Figure 4: Overall reaction between camphoric acid and zirconocene dichloride. R1 represents simple chain extension.
EC50 values for the metallocene dichlorides are generally a decade higher than for cisplatin for the breast cancer cell lines and for the standard W38 the difference is much greater. In comparison the EC50 values for the polymers are generally much less ranging from about a hundred fold less to greater than a thousand fold less compared to cisplatin, well within the nanogram/mL concentration range. Thus, the polymers show a greater toxicity towards the breast cancer cell lines compared to cisplatin. Further, the polymers exhibit a much enhanced inhibition towards the cancer cells compared to the Group 4 metallocene dichlorides. Since the Lewis base itself shows no inhibition towards any of the cells tested (to the concentration limits employed) it is the combination that is responsible for the activity.
There does not appear to be a difference for the metallocenes themselves and the polymers in the ability to inhibit growth for the two breast-associate cell lines.
The CI50 values for cisplatin are higher than for the metallocene dichlorides and CI50 values for the polymers are larger compared with the metallocene dichlorides. Further, the CI50 values for the polymers are also larger than CI50 values for cisplatin. In comparison to the metallocene dichlorides, the CI50 values for the polymers are generally a decade greater and in some cases over one thousand times larger.
In a number of studies involving Group 4 metallocenes we found that there is a trend with EC50 and CI50 values and the nature of the metals for the polymers. In general, the EC50 values follow the order of Ti>Zr=Hf and consequently the CI50 values follow the order Hf=Zr>Ti. The Zr and Hf values are similar while the values for the Ti are considerably different. As will be described shortly, while the titanocene dichloride was chosen for clinical studies, from our studies it would be the Zr and Hf polymers that should have been chosen. Chemically, it is known that this family of metals is the closest in chemical behavior of all of the families of elements to such an extent that it is difficult to separate the three metals.
Another concern involves the toxicity of the metal-containing moieties. The toxicity of the metal-containing units varies with the precise compounds as well as mode of measurement and test animal. For cisplatin the LD50 are as follows-Rat oral 25.8mg/kg; Rat Intravenous 8.0mg/kg; mouse oral 32.7mg/kg; and mouse intravenous 11 mg/kg (Pfizer Material Safety Data Sheet). For titanocene dichloride the LD50 values are rat intraperitoneal 25 mg/kg and mouse intravenous 180mg/kg (TCI America Material Safety Data Sheet). For zirconocene dichloride the rat LD50 intraperitoneal is 30mg/kg (TCI America Material Safety Data Sheet). For hafnocene dichloride the LD50 is given as intravenous mouse at 100mg/kg (TCI America MSD). While exact matches are not available, it appears that cisplatin has lower LD50 values, and is more toxic, than any of the metallocene dichlorides by about three fold.
Two metal-containing compounds have undergone clinical studies. The first is platinum and various derivatives similar to cisplatin [2]. This is now one of the most widely used anticancer drugs for treatment of solid tumors including breast cancer. The second one is titanocene dichloride [1]. Preclinical studies showed that titanocene dichloride inhibited the ovarian cancer cell line A2780 CP3. This cell line was twenty fold resistant to cisplatin but when exposed to titanocene dichloride it was only about two and a half resistant to titanocene dichloride. This is consistent with an absence of cross-resistance between the two metal containing drugs [41]. It is also in agreement with in vivo studies where titanocene dichloride showed much greater ability to inhibit cisplatin resistant human ovarian cancer xenografts compared to cisplatin. Titanocene dichloride largely overcame cisplatin resistance for the A2780CP and CH1cisR ovarian cancer cell lines in bcl-2 and p53 transfectants of A2780 cells [42].
Studies are consistent with DNA-metallocene interactions, including titanocene dichloride, zirconocene dichloride, and hafnocene dichloride, being a major determinant in the anticancer activity of these materials [43].
There is a difference between structural dependencies of our compounds and many metallocenes. It was concluded by some studies that the metallocene dichlorides themselves required liability of the chlorides for activity, but our compounds exhibit their activity as polymers rather than control delivery of small units [44-48]. Other studies have demonstrated anticancer activity when the halides are replaced so these conclusions are premature [45].
Titanocene dichloride underwent Phase I clinical trials. The trials indicated a dose-limiting side effect associated with nephrotoxicity and a number of unwanted physical side effects including nausea, reversible metallic taste, pain during infusion, hypoglycemia, with these features undesirable. Counter, the absence of an effect on proliferative activity of the bone marrow, generally a dose-limiting side effect, was positive. Some phase II clinical trials were undertaken with patients with breast metastatic carcinoma [47] and advanced renal cell carcinoma [48]. Unfortunately low activity discouraged further clinical study.
Table 4 contains EC50 and CI50 values for the monomers and polymers synthesized from reaction of camphoric acid and Group 15 triphenylmetal dihalides (Figure 5).
Table 4: EC50 Concentrations (micrograms/mL) for the tested compounds derived from reaction of Group 15 triphenylmetal dihalides and camphoric acid. Values given in ( ) are standard deviations for each set of measurements [49].
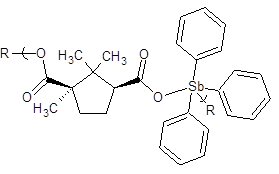
Figure 5: Repeat unit for the product of triphenylantimony dichloride and camphoric acid. R is simply chain extension.
Focusing only on the polymer results, the EC50 values are generally lower compared to the monomers and in the same range as the WI-38 values. The CI50 values are also not high. Of the three sets, the Group 15 polymers exhibit the least favorable EC50 and CI50 values and this is the reason that we are limiting our synthetic efforts with them.
In comparison with other metal/camphoric acid polymers, the metallocene polymers exhibit low EC50 values, to the nanogram/mL range, and CI50 values greater than one thousand for the hafnocene and zirconocene products. If this trend continues, for metal-containing polymers, the emphasis should be on the Group 4 metallocenes with respect to efforts to create anticancer drugs.
Summary
A variety of metal-containing polymers have been synthesized and their ability to inhibit two important breast cancer cell lines studied. In cases where the repeat unit for the polymer contains a O-phenylene unit, there is a difference in the ability of the polymer to inhibit the two cell lines. Polyethers that contain the O-phenylene structural unit showed good inhibition to the non-estrogen sensitive cell line, MDA-MB-231, but were less effective towards the MCF-7 cells, a well-characterized estrogen receptor positive control cell line. Consistent with many other polymers synthesized by us, the polymers showing the best activity towards the breast cancer cell lines were those derived from hafnocene and zirconocene dichloride, followed by organotin halides, and the least active overall were those containing the Group 15 metals, namely arsenic, antimony and bismuth.
References
- Abd-El-Aziz A, Carraher C, Pittman C, John E. Sheats, Martel Zelden. Macromolecules Containing Metal and Metal-Like Elements. Wiley-Interscience, Hobokin, NY. 2004.
- Roner MR, Carraher C. Cisplatin derivatives as antivirial agents. Inorganic and Organometallic Macromolecules. Springer, NY, NY. 2008.
- Carraher C. Antinomy-containing polymers. Inorganic and Organometallic Macromolecules. Springer, NY, NY. 2008.
- Carraher C. Condensation metallocene polymers. J Inorg Organomet Polymers. 2005; 13: 121-145.
- Carraher C. Organoantimony polymers. J Polym Mater. 2008; 25: 35-50.
- Carraher C, Roner MR. Organotin Polymers as Anticancer and Antiviral Agents. J Organomet Chem. 2014; 751: 67-82.
- Neto W, Pena L, Ferreira G, Fernando G, Fabricio M. Target delivery from modified polymers to cancer treatment. Current Org Chem. 2017; 21: 4-20.
- Conner E, Lees I, MacLean D. Polymers as drugs-advances in therapeutic applications of polymer binding. J Polym Sci A Polym Chem. 2017; 55: 3146-3157.
- Vogis D, Krishnan V, Mitragotri S. A review on engineering drug conjugates to improve combination chemotherapy. Colloid Interface Sci. 2017; 31: 75-85.
- Oh J. Polymers in drug delivery: chemistry and applications. Mol Pharm. 2017; 14: 2459.
- Connor E, Lees I, Maclean D. Polymer drugs-advances in therapeutic application of polymer binding agents. J Polym Sci A Polym Chem. 2017; 55: 3146-3157.
- Carraher C, Roner MR, Shahi S, Barot G. Structural Considerations in Designing Organotin Polyethers to Arrest the Growth of Breast Cancer Cells In Vitro . Materials. 2011; 4: 801-815.
- Carraher C, Roner MR. Organotin Polyethers as Biomaterials. Materials. 2009; 2: 1558-1598.
- Nebbia C, Dacasto M, Ceppa L, Gennaro Soffietti M, Spinelli P, Bergo V, et al. The Comparative Effects of Subchronic Administration of Triphenyltin Acetate (TPTA) on the Hepatic and Renal Drug-Metabolizing Enzymes in Rabbits and Lambs. Vet Res Commun. 1997; 21: 117-125.
- Siegmann-Louda D, Carraher C. Macromolecules Containing Metal and Metal-Like Elements. Wiley, Hobokin. 2004.
- Luo Y, Prestwich GD. Cancer-targeted polymeric drugs. Curr Cancer Drug Targets. 2002; 2: 209-226.
- Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010; 21: 797-802.
- Fang J, Nakamura H, Maeda H. Adv. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Drug Deliv Rev. 2011; 63: 136-151.
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011; 63: 131-135.
- Abdellaoui K, Boustta M, Morjani H, Manfait M, Vert M. Uptake and intracellular distribution of 4-aminofluorescin-labelled poly(L-lysine citramide imide) in K562 cells. J Drug Target. 1998; 5: 193-206.
- Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2- hydroxypropyl) methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin Cancer Res. 1999; 5: 83-94.
- Chourasia MK, Jain SK. Drug Deliv. Design and development of multiparticulate system for targeted drug delivery to colon. 2004; 11: 129.
- Fournier E, Passirani C, Colin N, Breton P, Sagodira S, Benoit JP. Development of novel 5-Fu-loaded poly (methylllidene malonate)-based microspheres for the treatment of brain cancers. Eur J Pharm Biopharm. 2004; 57: 189-197.
- Ulbrich K, Subr V. Polymeric Anticancer Drugs with pH-Controlled Activation. Adv Drug Deliv Rev. 2004; 56: 1023-1050.
- Goddard P, Williamson I, Brown J, Lusie E. Hutchinson, Julia Nicholls, Karel Petrak. Soluble Polymeric Carriers for Drug Delivery—Part 4: Tissue Autoradiography, and Whole-Body Tissue Distribution in Mice, of N-(2-Hydroxypropyl) Methacrylamide Copolymers Following Intravenous Administration. J Bioact Compat Pol. 1991; 6: 4-12.
- Phillips PC. Antineoplastic drug resistance in brain tumors. Neurologic Clinics. 1991; 9: 383-404.
- Carraher C, Doucetter R, Siegmann-Louda D. Inhibition of Human Ovarian Adenocarcinoma Cells by Organotin Polyamines Derived from 2-Chloro-1,4-benzenediamine in Dilute Aqueous HMPA and Aqueous DMSO Solutions. Polym Mater Sci Eng. 2004; 91: 569-570.
- Ulbrich K, Subr V. Polymeric Anticancer Drugs with pH-Controlled Activation. Adv Drug Deliv Rev. 2003; 56: 1023-1050.
- Carraher C. Introduction to Polymer Chemistry. Taylor and Francis, NY, NY. 2017.
- Carraher C. Polymer Chemistry. Taylor and Francis, NY, NY. 2018.
- Morgan P. Condensation Polymers by Interfacial and Solution Methods. Wiley-Interscience, NY, NY. 1965.
- Millich F, Carraher C. Interfacial Synthesis. Dekker, NY, NY. Vol I. 1977.
- Millich F, Carraher C. Interfacial Synthesis. Dekker, NY, NY. Vol II. 1977.
- Millich F, Carraher CE, Preston CJ. Interfacial Synthesis. Dekker, NY, NY. Vol III. 1977.
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Research and Treatment. 2004; 83: 249–289.
- Carraher C, Roner MR, Shahi K, Barot G. Structural consideration in designing organotin polyethers to arrest the growth of breast cancer cells in vitro . Materials. 2011; 4: 801-815.
- Barot G, Roner MR, Naoshima Y, Kazutaka Nagao, Kimberly Shahi, Charles E. Carraher Jr. Synthesis, structural characterization and preliminary biological characterization of organotin polyethers derived from hydroquinone and substituted hydroquinones. J Inorg Organomet Polymer Mater. 2009; 19: 12-27.
- Carraher C, Roner MR, Campbell A, et al. Synthesis of organotin polyesters from reaction of the salt of D-camphoric acid and organotin dihalides and initial anticancer activity. J Inorg Organomet Polym Mat. 2018; 28: 481-491.
- Hoch M. Organotin in the environment. Appl Geochem. 2001; 6: 719-743.
- Carraher, C, Roner MR, Campbell AG, Mosca F. Group IVB Metallocene Polyesters Containing Camphoric Acid and Preliminary Cancer Cell Data. International J Polymeric Materials Polymeric Biomaterials. 2018; 67: 469-479.
- Harstrick A, Schmoll H, Poliwoda H, Sass G, Rustum Y. Titanocendichloride activity in cisplatin and doxorubicinresistant human ovarian carcinoma cell lines. Eur J Cancer. 1993; 29: 1000-1002.
- Christodoulou CV, Eliopoulos AG, Young LS, Hodgkins L, Ferry DR, Kerr DJ. Anti-proliferative activity and mechanism of action of titanocene dichloride. Br J Cancer. 1998; 77: 2088-2097.
- Murray JH, Harding MM. Organometallic anticancer agents: the effect of the central metal and halide ligands on the interaction of metallocene dihalides Cp2MX2 with nucleic acid constituents. J Med Chem. 1994: 37: 1936-1941.
- Harding MM, Mokdsi G. Antitumour metallocenes: structureactivity studies and interactions with biomolecules. Curr Med Chem. 2000; 7: 1289-1303.
- Abeysinghe PM, Harding MM. Antitumour bis(cyclopentadienyl) metal complexes: titanocene and molybdocene dichloride and derivatives. Dalton Trans. 2007; 32: 3474-3482.
- Guang-Xiang L, Wang ML, Su, Li-Jun. Syntheses, Crystal Structures and Fluorescent Properties of Zinc Coordination Polymers Based on Camphor Acid and Imidazole Ligand. Wuji Huaxue Xuebao. 2011; 27: 1185-1187.
- Kroger N, Kleeberg UR, Mross K, Edler L, Hossfeld DK. Phase II clinical trial of titanocene dichloride in patients with metastatic breast cancer. Onkologie. 2000; 23: 60-62.
- Lummen G, Sperling H, Luboldt H, Otto T, Rübben H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemotherapy pharmacology. 1998; 42: 415-417.
- Carraher C, Roner MR, Mosca F, Slawek P. Synthesis and characterization, including cancer cell line inhibition, of Group VA (Group 15)-containing polyesters from reaction with camphoric acid. J Inorg Organomet Polym Mat. 2017; 27: 1627-1639.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.