- Research Article
- |
- Open Access
Comparison between HER2 extracellular domain in serum and HER2 overexpression in breast cancer tissue in the same patients
- Jamal Zidan;
- Oncology Institute, Ziv Medical Center, Safed, Israel
- Faculty of Medicine, Galilee, Bar-Ilan University, Safed, Israel
- Raneen Mzalbat;
- Oncology Institute, Ziv Medical Center, Safed, Israel
- Adi Sharabi;
- Oncology Institute, Ziv Medical Center, Safed, Israel
- Moshe Stein;
- Department of Oncology, Rambam Medical Center, Haifa, Israel
- Osamah Hussein
- Department of Oncology, Rambam Medical Center, Haifa, Israel
- Department of Internal Medicine, Ziv Medical Center, Safed, Israel

| Received | : | Oct 17, 2019 |
| Accepted | : | Dec 12, 2019 |
| Published Online | : | Dec 14, 2019 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Zidan J, Mzalbat R, Sharabi A, stein M, Hussein O. Comparison between HER2 extracellular domain in serum and HER2 overexpression in breast cancer tissue in the same patients. Ann Breast Cancer. 2019; 2(1): 1012.
Abstract
Background: Human epidermal growth factor receptor 2 (HER-2) is an important prognostic and predictive factor in women with breast cancer (BC). The importance HER-2 extracellular domain (ECD) in serum is not yet determined. The aim of this study was to explore the correlation between serum ECD and tissue HER-2 expression and to compare ECD levels with clinical and pathological features in primary BC patients.
Methods: In this prospective study only patients with stage I–III BC were included. Serum ECD levels were measured by ADVIA Centaur automated assays before surgical resection of the tumor. Serum ECD >15ng/ml was considered to be positive.
Results: Eighty patients with breast tumors were included. Stage I–III BC was diagnosed in 64 patients, Ductal carcinoma in situ in 9 and benign tumors in 7 patients. HER-2 overexpression was observed in 8 of 64 patients (16.4%). Mean value of serum ECD was 10.9 ng/ml (range: 6.7 to 21.5). Four (6.2%) of the 64 patients had high ECD levels and in 60 (93.8%) patients lower levels of ECD were found. No significant relationship was found between ECD levels and tissue HER2 overexpression. ECD was higher in women aged > 40 than in women aged < 40. No significant relation was found between ECD and all clinical and pathological features.
Conclusion: The sensitivity of HER2 ECD for the diagnosis of HER2 overexpression in primary breast cancer is poor. No significant correlation was found between ECD levels and tissue HER2 expression, clinical and pathological characteristics of primary BC.
Keywords: Serum ECD; HER2 in tumor; Primary breast cancer; Comparison
Introduction
Breast cancer (BC) is the most common malignancy in European, North American and Israeli women [1,2]. A multimodal therapy is used for the treatment of BC which includes: surgery, radiotherapy, chemotherapy, hormone therapy and an increasing number of biological targeting therapies. BC rate of cure depends on many factors; tumor size, lymph nodes metastases, distant metastases, age of patients, ER and PR status, and HER2 expression [3-6]. HER2 has also a predictive value for treatment with biological targeting agents as trastuzumab and lapatinib when it overexpressed in BC [7]. The c-erbB-2 oncogene encodes a 185 kilodalton (KD) epidermal growth factor receptor-like membrane glycoprotein (HER-2) with tyrosine kinase (TK) activity [8]. Gene amplification and overexpression of HER-2 occurs in approximately 20-25% of breast carcinomas [9]. The most commonly used method for the detection of HER-2 in tumor tissue is immunohistochemical (IHC) test, fluorescent in situ hybridization (FISH) test, chromogenic in situ hybridization (CISH) test and other methods [6]. The extracellular domain (ECD) of HER-2, such as the ECD of other HER family members, is marked by two consensus, cysteine-rich motifs [10]. It may be cleaved, shed from the surface of BC cells and released into the serum [11]. This ECD fragment is generated by proteolytic cleavage of full-length HER-2 by tissue metalloprotienases (TMPs) [11-13], which results in shed ECD and a membrane-bound, N-terminally truncated HER-2 fragment with in vitro TK activity (p95 HER-2) [14,15]. ECD production is increased by TMP activators and is decreased by TMP inhibitors in vitro [13,16]. Biochemical studies using immunoprecepitation, Western blot procedures MoAb specific for the HER-2 oncoprotein, demonstrated that the ECD is a glycoprotein with a molecular weight between 97 KD and 115 kd [11]. It was shown that the circulating HER-2 ECD could be detected in the plasma of healthy subjects and may be elevated in patients with primary breast cancer (PBC) and metastatic breast cancer (MBC) [16,17]. These observations were confirmed in serum samples [17]. HER-2 ECD levels can be measured in serum. While determination of tissue HER-2 by IHC or CISH is a one-time event, serum ECD can be measured any time during follow-up [12,15].
Serum HER-2 ECD levels may predict tumor HER2 status as detected by IHC. The utility of serum ECD values as baseline and during therapy as a potential marker for both evaluation of HER-2 overexpression and as a potential marker for tumor response or progression is currently a matter of debate [18]. The ECD can undergo shedding in up to 30% of cases, while elevated ECD concentrations are observed in 3-12% of primary breast cancer PBC patients [19]. Mostly there was no concordance between ECD serum levels and HER-2 expression in PBC tissue [18]. This issue is not totally clear nor accepted in the literature.
High ECD serum levels may be associated with poor prognosis and may also be used as a detective marker for biological targeting therapy of BC [11]. There is no consensus in the literature about the concordance between serum ECD levels and HER-2 expression in PBC tissue. Some observations suggest that alteration in serum ECD could be useful in diagnosis and followup of BC. Other studies showed no correlation between ECD serum levels and HER-2 overexpression in tumor tissue [18,19].
The aim of this study to explore prospectively the concordance between circulating HER-2 ECD levels in serum and HER-2 overexpression measured by IHC and CISH in tumor tissue of women with PBC (primary, localized non-metastatic disease).
The second aim is to compare ECD levels with clinical and pathological characteristics. This would have important clinical implications because ECD would then provide a convenient surrogate for HER-2 tissue diagnosis especially in recurrent disease.
Patients and methods
Patients Population and Samples Collection
Ethic committee approval was achieved for this study. Patients provided written informed consent before enrollment. Blood samples from 100 newly diagnosed women who underwent surgery because they were thought to have operable breast cancer without metastases were taken prior to the operation. Only true cut biopsy or fine needle aspiration was permitted before surgery for tumor resection. Blood samples were centrifuged within 20 minutes after blood collection at 350 rpm for 10 minutes at room temperature. After centrifugation, serum was stored in minus 70˚C until measurement. Women were operated at Ziv Medical Center between January 2008 and December 2010. Staging was done according to the American Joint Committee on Cancer (AJCC) classification [20].
Estrogen receptor (ER), progesterone receptor (PR) and HER2 status were assessed at the time of surgery on FFPE tissue blocks of the primary tumor. Recorded clinical and pathological features for each patient include age, histology, ER and PR status, tumor stage, tumor grade and hormonal therapy in the past. HER-2 evaluation in tissue samples was done by IHC and in cases of HER-2 plus 2 CISH test was done.
IHC
IHC testing was performed using the HercepTest (DakoCytomation, Carpinteria, CA), following the manufacturer’s instructions. Briefly, this procedure includes deparaffinization and rehydration steps, followed by an epitope retrieval step in which the tissue sample is incubated in EDTA buffer solution at more than 95°C for 20 minutes. The slide is then subjected to a series of alternating washes in Phosphate buffered saline buffer (PBS) and incubation steps beginning with first a peroxidase-blocking reagent for 10 minutes and followed by a polyclonal antibody to HER2 (A0485; Dako) applied at a 1:200 dilution in PBS to sections and incubated for 60 min at room temperature further followed by a visualization reagent (dextran polymer) conjugated with horseradish peroxidase for 30 minutes , and finally with a 3,3'-diaminobenzidine chromogen solution for 10 min. After a final wash, the slide is counterstained with hematoxylin.
Tumor cells with circumferential membranous positivity were considered as HER2 protein over expression, and scoring was performed according to the manufacturer's recommendations by pathologists that had experience in breast pathology and immunohistochemical interpretation of HER2 testing. According to the Hercept Test criteria, immunoreaction was scored as: 3+ if >10% of tumor cells showed strong and complete membrane staining, 2+ if membrane positivity was moderate and complete in >10% cells, 1+ if membrane positivity was weak and incomplete in >10% cells and 0 if membrane staining was absent or present in <10% cells. Tumors scored as 3+ were considered HER2 positive (overexpressed) while tumors scored as 0/1+ were designated as HER2 negative. In 2+ tumors evaluated by IHC, CISH analysis was carried out.
CISH
CISH allows detection of gene amplification, chromosome translocation and chromosome number using conventional peroxidase reactions under bright field microscope on FFPE tissues (4–5 mm thickness). The essence of CISH is the ability of labeled nucleic acid probes to hybridize, in situ, to specific sections of complementary nucleic acid in the sample. Probe hybridization results may be visualized within the context of the surrounding tissue morphology. Therefore, pathologists can view tissue morphology and gene aberrations simultaneously [17]. The Zymed’s 84-0146 SPoT-Light1 HER2 CISHTM Kit was used. CISH staining results may be clearly visualized with a standard bright field microscope and a 406 dry lens. Tumors with HER2 gene amplification typically appear as a large peroxidase-positive intranuclear gene copy clusters, as numerous individual peroxidase-positive small signals, or as a mixture of clusters and individual gene copies. Tumors with normal HER2 gene status typically exhibit two signals or dots per nucleus.
Serum HER2 ECD assays
For serum HER2 ECD concentration testing the ADVIA Centaur automated assays is used. ADVIA Centaur HER2/neu assay is two-site sandwich immunoassay using direct, chemiluminescent technology. The Light Reagent is composed of the monoclonal mouse antibody TA-1, labeled with acridinium ester. The Fluorescein Conjugate Reagent is composed of the monoclonal mouse antibody, NB-3, labeled with fluorescein. These two monoclonal antibodies are specific for unique epitopes on the ECD of HER2/neu. The Solid Phase is composed of purified anti-fluorescein monoclonal mouse capture antibody, which is covalently coupled to paramagnetic particles. The sample is incubated with Fluorescein Conjugate Reagent and Light Reagent simultaneously for 5.5 minutes. After this incubation, the Solid Phase is added and the mixture is incubated for an additional 2.75 minutes. After this final incubation, the immuno-complex formed is washed with water prior to initiation of the chemiluminescent reaction.
A direct relationship exists between the amount of HER2/ neu present in the patient sample and the amount of relative light units (RLUs) detected by the system. For quality control of the ADVIA Cenatur HER2/neu assay, HER2/neu equivalent quality control material will be used. According to the target value sheet for the suggested Expected Values.
Statistical analysis
All analyses were carried out using SPSS Data Analysis Program (ver. 17.1). A Students T test was used to assess for differences in serum ECD levels between the groups. Chi-squared contingency tables were used to assess an association between detection of ECD in serum and tissue. Chi-squared test was also used to assess the association among clinical–pathological features and the expression of HER2 tissue or the levels of HER2 ECD.
In order to determine high and low levels of HER2 ECD in the serum 2 cutoffs for positivity were taken in consideration, 15ng/ mlas accepted by most studies in the literature and 12ng/ml for comparison [12,19,21].
Results
A total of 80 women were included in this study. In 20 women no malignant or benign tumors were found. All patients have a localized tumor in the breast; 64 women (80%) with invasive BC, 9 women (11.2%) with Ductal Carcinoma in situ (DCIS) and 7 women (8.8 %) with benign breast disease. Pathological distribution presented in (Table 1).
Among the 64 patients with BC; 19 (29.7%) patients had stage I, 36 (56.3%) had stage II and 9 (14%) had stage III disease. Mean age at diagnosis was 57.9 years (range: 31-87). Forty nine (77.5%) women were Jewish and 15 (22.5%) were Arabs (Table 2). Forty six (71.9%) had ER positive and 42 (65.6%) had PR positive. Histological grade I was found in 15 (23.4%) tumors, grade II in 33 (51.6%) and grade III in 16 (25%) of the tumors.
Table 2: ECD levels compared to clinical and pathological characteristic in 64 patients with invasive breast cancer.
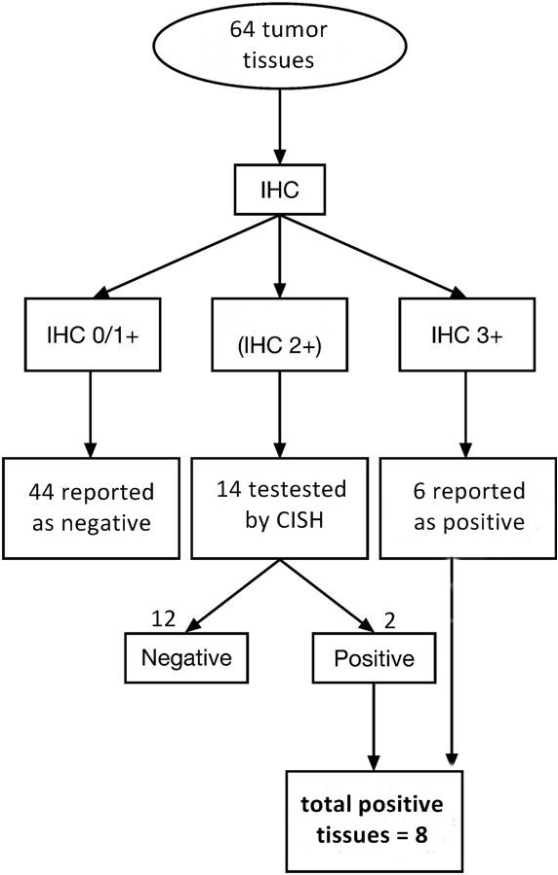
Tissue HER-2 status: HER-2 was detected in the 64 tumor tissues of the BCs by IHC test and/or CISH test according to HER2 algorithm recommendations. Forty four patients were HER-2 IHC 0/1+; reported as negative and 6 (%) patients with 3+ reported as HER-2 positive. Fourteen patients with HER2: 2+ by IHC were retested by CISH and 2 of them had HER-2 amplification by CISH and reported as HER-2 positive. A total of 12.5% of patients had overexpression of HER-2 in tissue tumor samples (Figure 1).
Figure 1: Tissue HER2 status and test used for detection. IHC: immunohistochemistry, CISH: chromogenic in situ hybridization.
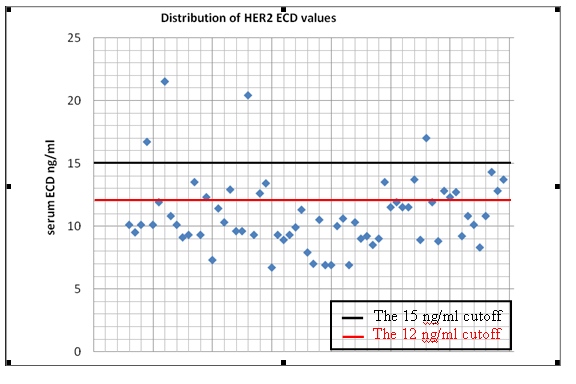
Serum HER2 ECD status: The mean value of ECD was 10.9 ng/ml ( range 6.7 - 21.5 ng/ml). With a cutoff value of 15 ng/ ml 4 (6.2%) of the 64 patients with BC had preoperative HER-2 ECD high levels while 60 (93.8%) patients had ECD low levels (< 15 ng/ml). (Figure 2).
Every point represents one different serum sample. The values were ranged between 6.7 and 21.5 ng/ml. At the 15ng/ ml cutoff, 4 samples were positive.
Comparison between serum ECD levels and tissue HER2 status: The bivariate distributions of patients with tissue HER2 status and serum ECD levels are shown in table 2. We observed high serum ECD levels in one (12.5%) of 8 patients with tissue HER-2 positive and in 3 (5.4%) of 56 patients with tissue HER2- negative status. No concordance between high ECD levels and tissue HER-2 status was found (P: 0.446) (Table 2). Mean value of ECD in serum of patients with positive HER-2 in tissue was 10.3 ng/ml (range: 6.9- 16.) and 11 ng/ml (range: 6.7-21.5) in patients with negative HER2 in tissue (P: 0.380).
Discussion
Mostly tissue samples for HER2 evaluation are taken from the primary tumor. In MBC generally HER2 detection is done by using formalin embedded paraffin blocks. Rarely a biopsy is done from the metastatic sites for HER2 evaluation, although a discordance was reported to be up to 25% between HER2 overexpression in the primary versus metastatic tissue in the same patients [22,23].
ECD evaluation is done from serum of patients in any period and stage of the disease and in some studies from long stored blood samples. Concordance between serum ECD levels and HER2 in tissue may makes this test useful for treatment decision in primary or metastatic disease without the need for biopsy for HER2 evaluation. The presence of ECD protein in the serum is dependent upon leakage of the protein into the circulation. This process may to be influenced by a number of physical factors including protein production and release from the cell, tumor architecture, cell orientation, and development of tumor vascularity [21-23].
In PBC, HER2 ECD has been detected in up to 30% of patients [16,17], but the clinical significance of circulating ECD is uncertain and the relationship of baseline HER2 ECD levels with tissue HER2 overexpression in primary tumors has been little studied. The role of ECD in patients with PBC as prognostic factor also is not well defined. The percentage of elevated serum levels ECD are extremely variable in PBC patients at the time of diagnosis. In our study, high ECD levels were observed in 6.2% (4/64) (cutoff of 15 ng/ml) of PBC patients; similar to the results reported by other authors [24,25] (Table 2).
Tumor burden is important because normal cells and tumor cells which not have HER2 overexpression contain also HER2. If bulky tumor or bulky metastases are present (big tumor burden) ECD levels may increase (false positive) although HER2 is not overexpressed in tumor tissue [26]. Concordance or discordance between ECD levels in serum and HER2 expression in tumor tissue in PBC can be more representative since tumor size is smaller and patients have no macroscopic metastases. Most, if not all of the ECD could be excreted by the primary tumor [25,27].
The similar prevalence rates of serum and tumor tissue HER2 positivity among patients with early BC suggest that there might be a strong correlation. This would have important clinical implications, as ECD would then be used for follow-up and treatment decision with trastuzumab when biopsy is not available from the primary tumor and metastases. However most studies found no correlation between serum ECD and tissue HER2 status among women with newly diagnosed BC [27-29]. The percentage of elevated serum ECD levels are extremely variable in PBC patients at the time of diagnosis [29-34]. Few studies showed an association between high ECD levels and tissue HER2 status. HER2 ECD levels were moderately concordant with tissue HER2 status [33]. Data from 63 clinical studies incorporating serum HER2 ECD measurements are inconsistent [34]. Comparison of results between studies is problematic because of an absence of standardization in the studies reviewed; 15 different cutoff s were used to distinguish between normal and raised serum concentrations, with at least13 different assay techniques [34]. The number of patients and the stage of their breast cancer varied substantially. Furthermore, some studies were designed prospectively, whereas others were retrospective. Consequently, any general conclusions drawn from existing data are made in the context of substantial inter-study variation, and a comprehensive systematic metaanalysis to account for these variations is unlikely to resolve the question of the usefulness of HER2 ECD measurements [28-34]. The discordance between ECD and HER2 in tissue may be attributable in part because in some studies serum was collected pre while in others postoperatively. In some studies ECD was determined in long stored blood samples (can be years) of patients participated in different treatment studies [35].
In the recent study no concordance was found between serum ECD levels and HER2 overexpression in PBC. Mean ECD value in serum of patients with HER2 positive tissues was 10.3 ng/ml and in patients with HER2 negative tissues was 11 ng/ ml. Our study is a prospective one and all blood samples were taken one day before biopsy or excision of PBC, immediately centrifuged for 10 minutes and stored in a minus 70 C until the ADVIA Centaur automated test was done, in order to avoid all technical deficiencies mentioned above in other studies.
We found poor sensitivity of HER2 ECD for the diagnosis of HER2 positive PBC. In addition some proportion of HER2 negative tumors can produce ECD. We used a cutoff of >15 ng/ml of ECD positivity as accepted by most studies in the literature.
For comparison we also repeated our calculations using a cutoff of >12ng/ml. No change in ECD concordance with HER2 tissue was observed using these different cutoffs. Based on this study there is no evidence to support the use of ECD to determine HER2 status in PBC. The question remains opened regarding the use of ECD in MBC.
Moreover, in our study, levels of ECD were not associated with several factors related to tumor aggressiveness, such as stage of primary tumor, tumor size, lymph node involvement, poor histological differentiation, ER negativity and PR negativity. Interestingly there was a trend for higher mean ECD levels in Arab women with PBC compared to Jewish women with PBC (p=0.097). It was reported that BC have more aggressive features in Arab versus Jewish women in Israel [34]. This may explain this trend, since other studies found an elevated number of patients with high ECD (>15ng/ml) in stage III BC and in tumors with poorly differentiated histology [33].
In the recent study ECD was detected in 9 women with ductal carcinoma in situ. Mean ECD level was 10.4 ng/ml (range: 7.7- 14.2). In 7 women with benign breast disease mean ECD level was 10.7 ng/ml (range: 6.7-12.6). There is no consensus in the literature on the importance of HER2 elevation in tissue or serum in patients with DCIS or benign breast diseases.
It appears that elevated ECD serum levels were seldom detected in early stage BC even though the primary cancer tissue was HER2 positive. Elevated serum ECD before resection of the PBC may be used as a prognostic factor and for monitoring of response to treatment [36-40].
Conclusion
There was no concordance between ECD levels in serum and HER2 expression in cancer tissue in patients with PBC. The sensitivity of ECD for the diagnosis and follow up of HER2 positive primary BC is poor. No correlation was found between ECD levels and the different clinical and pathological features of PBC tumors. Due to our results HER2 ECD cannot be used to determine HER2 status in primary BC.
References
- American Cancer Society. Breast cancer facts and figures 2005- 2006.
- The israeli ministry of health website :http://www.health.gov. il/
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005; 97: 1180-1184.
- Schnitt SJ. Estrogen receptor testing of breast cancer in current clinical practice: whats the question? J Clin Oncol. 2006; 24: 1797-1799.
- Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer-histopathological and prognostic considerations. Br J Cancer. 1997; 75: 1318-1323.
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, et al. American Society of Clinical Oncology/ College of American Pathologists guideline recommendations for human epidermal growth factor 2 testing in breast cancer. J Clin Oncol. 2007; 25: 118-145.
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986; 232: 1644-1646.
- Rabindran SK. Antitumor activity of HER-2 inhibitors. Cancer Lett. 2005; 227: 9-23.
- Lovekin C, Ellis IO, Locker A, Robertson JF, Bell J, et al. c- erbB-2 oncoprotein overexpression in primary and advanced breast cancer. Br J Cancer. 1991; 63: 439-443.
- Massagué J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990; 265: 21393–21396.
- Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185 is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991; 266: 1716-1720.
- Liu PC, Liu X, Li Y, Covington M, Wynn R, et al Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther Epub. 2006; 5: 657-664.
- Yuan CX, Lasut AL, Wynn R, Neff NT, Hollis GF, et al. Purification of Her-2 extracellular domain and identification of its cleavage site. Protein Expr Purif. 2003; 29: 217-222.
- Segatto O, King CR, Pierce JH, Di Fiore PP, Aaronson SA. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 2006; 8: 5570-5574.
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin. Nature. 2003; 421: 756-760.
- Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadateactivable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999; 59: 1196-1201.
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene. 1993; 8: 2917-2923.
- Leary AF, Hanna WM, van de Vijver MJ, Penault-Llorca F, Rüschoff J, et al. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009; 27: 1694-16705.
- Isola JJ, Holli K, Oksa H, Kallioniemi OP. Elevated c-erbB-2 oncoprotein levels in preoperative and follow-up serum samples define an aggressive disease course in patients with breast cancer. Cancer. 1994; 73: 652-658.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. AJCC cancer staging manual, 7th ed. 2009.
- Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, et al. Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002; 20: 1467- 1472.
- Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005; 93: 552-556.
- Cardoso F, Di Leo A, Larsimont D, Gancberg D, Rouas G, et al. Evaluation of HER2, p53, bcl-2, topoisomerase II-alpha, heat shock proteins 27 and 70 in primary breast cancer and metastatic ipsilateral axillary lymph nodes. Ann Oncol. 2001; 12: 615-620.
- Isola JJ, Holli K, Oksa H, Kallioniemi OP. Elevated c-erbB-2 oncoprotein levels in preoperative and follow-up serum samples define an aggressive disease course in patients with breast cancer. Cancer. 1994; 73: 652-658.
- Saghatchian M, Guepratte S, Hacene K, Neumann R, Floiras JL, et al. Serum HER-2 extracellular domain: relationship with clinic biological presentation and prognostic value before and after primary treatment in 701 breast cancer patients. Int J Biol Markers.2004; 19:14-22.
- Carney WP, Neumann R, Lipton A, Leitzel K, Ali S, et al. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast cancer. Clin Chem. 2003; 49:1579-1598.
- Willsher PC, Beaver J, Pinder S, Bell JA, Ellis IO, et al. Prognostic significance of serum c-erbB-2 protein in breast cancer patients. Breast Cancer Res Treat. 1996; 40: 251-255.
- Quaranta M, Daniele A, Coviello M, Savonarola A, Abbate I, et al. c-erbB-2 protein level in tissue and sera of breast cancer patients: a possibly useful clinical correlation. Tumori. 2007; 92: 311-317.
- Salvadori B, Pinzani P, Distante V, Casella D, Bianchi S, et al. Comparison of pre- and postsurgical concentrations of blood HER-2 mRNA and HER-2 extracellular domain reflects HER-2 status in early breast cancer. Clinical Chemistry. 2005; 51: 254- 256.
- Krainer M, Brodowicz T, Zellinger R Wiltschke C, Scholten C, et al. Tissue expression and serum levels of HER-2/neu in patients with breast cancer. Oncology. 1997; 54: 475-481.
- Pallud C, Guinebretiere JM, Guepratte S, Hacene K, Neumann R, et al. Tissue expression and serum levels of the oncoprotein HER-2/neu in 157 primary breast tumours. Anticancer Res. 2005; 25: 1433-1440.
- Leitzel K, Chaudri-Ross HA, Evans DB, Ali SM, Demers L, et al. Elevated pretreatment plasma HER2/neu and decreased response to neoadjuvant letrozole and tamoxifen. Proc Breast Cancer Symposium. 2007; 113: 108.
- Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008; 19: 883-890.
- Leyland-Jones B, Smith B. Serum HER2 testing in patients with HER2-positive breast cancer: the death knell tolls. Lancet Oncol. 2011; 12: 286-295.
- Zidan J, Sikorsky N, Basher W, Sharabi A, Friedman E, et al. Differences in pathological and clinical features of breast cancer in Arab as compared to Jewish women in Northern Israel Int. J. Cancer. 2012; 131: 924-929.
- Moreno-Aspitia A, Hillman D, Dyar S, Tenner K, Gralow J, et al. Soluble Human Epidermal Growth Factor Receptor 2 (HER2) Levels in Patients With HER2-Positive Breast Cancer Receiving Chemotherapy With or Without Trastuzumab. Results From North Central Cancer Treatment Group Adjuvant Trial N9831. Cancer. 2013; 119: 2675-2682.
- Lee S, Lee J, Yu J, Ko B, Kim H, et al. Preoperative serum HER2 extracellular domain levels in primary invasive breast cancer. BMC Cancer. 2014, 14: 929.
- Zhang L, Riethdorf S, Wu G, Wang T, Yang K, et al. Meta-Analysis of the Prognostic Value of Circulating Tumor Cells in Breast Cancer. Clin Cancer Res. 2012; 18: 5701-5710.
- Reix N, Malin C, Chenard M-P, Bellocq J-P, Delpous S, et al. A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res Treat. 2016: 160: 249-259.
- Lam L, Czerniecki B, Fitzpatrick E, Xu S, Schuchter L, et al.Interference-Free HER2 ECD as a Serum Biomarker in Breast Cancer. J Mol Biomark Diagn. 2013; 4: 3.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.