- Research Article
- |
- Open Access
“Analysis of the Relationship of the Grades of Invasive Ductal Carcinoma with the Ki-67 Index in Breast Cancer Patients and Its Application in Third World Countries”
- Jyoti Taneja*;
- Department of General Surgery, Dr NC Joshi Memorial Hospital (Govt of NCT), Delhi, India.
- Viraj Borgaonkar;
- Department of General Surgery, Seth Nandlal Dhoot Hospital, Aurangabad, Maharashtra, India.
- Vijay Borgonkar;
- Department of General Surgery, Seth Nandlal Dhoot Hospital, Aurangabad, Maharashtra, India.
- Vikas Agrawal;
- Department of General Surgery, Seth Nandlal Dhoot Hospital, Aurangabad, Maharashtra, India.
- Prassana Somvanshi
- Department of Plastic Surgery, Grant government medical college and Sir JJ group of hospitals, Mumbai, Maharashtra, India.

| Received | : | Nov 16, 2021 |
| Accepted | : | Dec 24, 2021 |
| Published Online | : | Dec 27, 2021 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Taneja J, Borgaonkar V, Borgonkar V, Agrawal V, Somvanshi P. “Analysis of the Relationship of the Grades of Invasive Ductal Carcinoma with the Ki-67 Index in Breast Cancer Patients and Its Application in Third World Countries”. Ann Breast Cancer. 2021; 4(2): 1021.
Keywords: Breast cancer; KI-67index; IDC grades.
Abbreviations: IDC: Invasive Ductal Carcinoma; PR: Progesterone Receptor; ER: Estrogen Receptor; HER2: Human Epidermal Growth Factor Receptor 2; IHC: Immunohistochemistry; HPE: Histopathology Examination; FNAC: Fine Needle Aspiration Cytology; PCNA: Proliferating Cell Nuclear Antigen; EBC: Early Breast Cancer; DSS: Disease Specific Survival; DFI: Disease Free Interval; TNBC: Triple Negative Breast Cancer.
Abstract
Background and objectives
We aim to analyse the relationship of the grades of Invasive Ductal Carcinoma with the KI-67 index in breast cancer patients and, stratifying patient prognosis and to create a comprehensive prognostic index for clinical applications. A mono-institutional cohort for cancer breast patients having complete clinical, radiological, histological, and follow-up data. The <20% and >20 % Ki67 cut-offs were correlated to IDC grades, lymph-node positivity and tumor size. Patients with tumors with Ki-67 > 20% showed the poorest prognosis. In addition, the tumor size, number of metastatic lymph nodes and the Ki-67 > 20% was given a score value, depending on definite cut-offs and used to create a prognostic index, which was applied to the population. We confirm that the 20% Ki67 cut-off is significant to differentiate high-risk patients in breast cancers, and it is suggested to integrate it with other prognostic factors, to better stratify patients at risk of adverse outcome.
Methods
Study design and populationWe investigated 200 females with primary breast cancers who underwent breast surgery from JANUARY 2013 to MARCH 2018 at the Breast Unit of the Seth Nanadlal hospital, Aurangabad, Maharashtra.
Ethical approval for this study was obtained from the Ethical Committee of our Institution. The clinico-pathological data’s were obtained from clinical charts: age at diagnosis, type of surgery (conservative surgery vs modified radical mastectomy), therapy given, type of breast cancer, and site of recurrence. In addition, tumor size of (<3 vs. ≥3 cms), histological grade and type of tumor, numbers of nodes involvement, Progesterone Receptor (PR), ER, HER2, and Ki-67 was obtained from pathological reports.
Statistical analyses: Pearson’s Chi-square test was preliminary performed. A model was created for evaluating the prognostic role of different variables. p values <0.05 were considered significant. Statistical analyses were performed using SSPS 20th version.
Sample size being 233 by formula 4*p*q/E2 But due to the exclusion of 50 patients and time constraints we could only evaluate 200 patients in our study.
Results
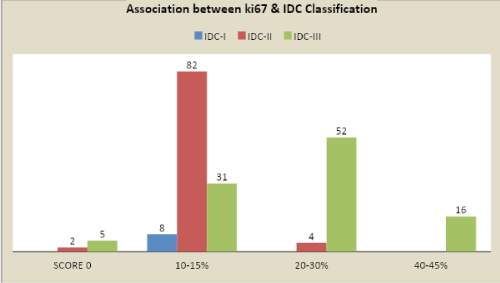
Higher values > 20% of ki67 we can almost predict 95% that the grade of the the tumor is IDC grade III and poorer is the prognosis and proliferation of the tumor is more.
But, on the contrary we also observed that in IDC grades I/II usually, have KI-67 <20 %.
With almost 80 % accuracy, that higher is the proliferating index with the poor disease free interval and overall survival rates.
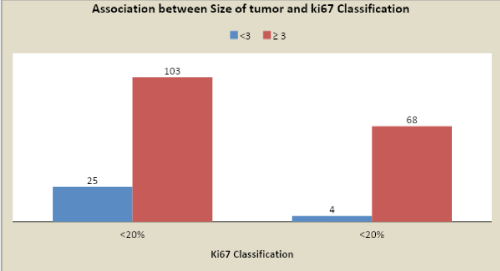
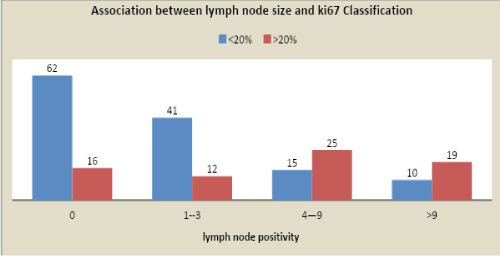
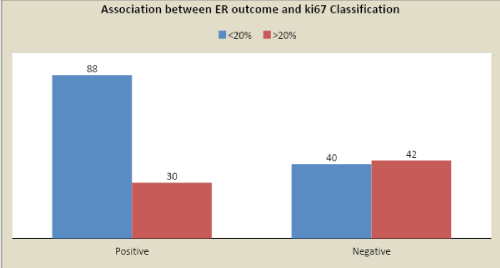
The <20% and >20 % Ki67 cut-offs were correlated to IDC grades (P<0.0001), lymph node positivity (P<0.0001) and tumor size (P=0.007), ER + (P<0.0001). Patients with tumors with Ki67 > 20% showed the poorest prognosis. Moreover, to tumor size, the number of metastatic lymph nodes and Ki67 > 20% was given a score value, varying depending on definite cut-offs and used to create a Prognostic index, which was applied to the population.
Conclusion: In conclusion, we confirm the reliability of Ki67 as a prognostic marker in breast cancers, using a cut-off value of 20 %, and we stress the important role of Ki67 in the clinical management of patients. It is easily correlated with grades of IDC explaining greater the value of ki-67 higher is the Bloom Richardson’s score with poorer prognosis.
We can practically advocate that the use of prognostic index for breast cancer could “proxy” the multigene or other prognostic test results in third world countries.
Introduction
Ki-67 is one of the most debatable and discussed proliferation tissue marker in breast cancer for the diagnostic studies and for the treatment decisions in breast cancer patients. Breast cancer is a diverse disease with various histological subtypes. Different types exist which can be defined by either genetic array or IHC analyses [1]. In the multigene tests, proliferation has a great impact on predicting the recurrence [2,3]. Similarly, in addition to the HPE, the evaluation of Ki67 is one of the significant prognostic factor in breast cancer patients [4]. A variety of techniques are available to assess proliferation of the tumor cell such as calculating mitotic figures in the tissue sample , flow cytometry to check for the percentage of cells being in the S phase , thymidine-labeling index, Proliferating Cell Nuclear Antigen (PCNA), or cyclins E and D [5,6,7].
One of the significant method is analysis of Ki-67 antigen. It is a prognosticating factor for breast cancer that has been investigated in many retrospective and prospective studies. It is well known fact that the Ki-67 nuclear antigen is expressed only in few phases of the cell cycle S, G1, G2, and M phases, but it is never expressed in G0 [8,9]. However, till today there is no acceptable definite cut-off definition for Ki-67 [10,11].
The purpose of this study is to evaluate the value of Ki-67 as a prognostic marker and to analyse the associations between Ki-67 and Invasive ductal carcinoma in the routine clinical setting in a third world country setting ,where most of the patient cannot afford the cost and government funding’s are usually insufficient to cover sizeable population.
Grades of IDC
Scarff-Bloom-Richardson grading system. Scoring system indicates that, three factors are
Taken into account by a pathologist:
The amount of gland formation (“differentiation”).
The nuclear features (“pleomorphism”).
The mitotic activity (division rate of tumor cell).
Grade 1 tumors have a score of 3-5.
Grade 2 tumors have a score of 6-7.
Grade 3 tumors have a score of 8-9.
Material and methods
Patients
This analysis to be carried out from the history charts of patients diagnosed with cancer breast and who attended the surgical out-patient department of a tertiary care hospital like Seth Nandlal Dhoot Hospital, Aurangabad. A detailed past history with respect to age of menarche ,age at the time of first birth, breast feeding, permanent or temporary contraception, family history of malignancy.
A detailed preoperative evaluation of suspected cancer breast patients was confirmed by triple assessment including detailed history and examination, radiological imaging and tissue diagnosis. This analysis was used to ascertain the prevalence and distribution of different grades of IDC of breast cancer and its aggressiveness. Aggressiveness of the cancer was assessed by the Grade of tumour and the Lymph node status on Histopathological examination. Bias with respect to false information given by patients is anticipated and was accepted.
Study site and study population
All the cases of breast cancer were obtained from the medical records of the hospital.
After obtaining the diagnosed cases of breast, cancer medical records were reviewed.
Presenting symptoms and signs with associated risk factors were obtained retrospectively from patient history.
Radiological findings with the histopathological reports were obtained from the medical records of the patient who were subjected to surgery (Modified radical mastectomy) or other breast conserving surgeries.
Results of ki-67, ER/PR, HER-2/NEU.
Study design
Sample size and sample techniqueConsidering the prevalence of breast cancer to be 30% in a tertiary care
Hospital in Aurangabad [12], the sample size will be 233 by formula 4*p*q/E2
Where, Formula: 4*0.3*0.7/0.062
p - Prevalence (0.3).
q - 1-prevalence (0.7).
E- Allowable error (10 or 20% of prevalence) here, 20% of 0.3 is 0.06.
Sample size and time frame233 by formula 4*p*q/E2
Cancer breast patients treated under our surgery department between January 2013 To March 2018.
Selection CriteriaInclusion criteria: All the primary cases of Invasive Ductal carcinoma.
Exclusion criteria: Any cancer breast other than IDC.
Inflammatory breast cancer
Paget disease of the nipple
Phyllodes tumor
Angiosarcoma
Ductal carcinoma in situ
Recurrent breast cancer patients
Patients with missing data
Patients with missing data
Methodology
In the duration of the study (January 2013 To March 2018) all the cases diagnosed and treated for cancer breast were included.
Total number of patients for breast cancer were 250. Patients were selected on the basis on HPE reports of IDC strictly. Thus, our study sample size came out 200 after exclusions.
Ethical approval for this study was obtained from the Ethical Committee of our Institution.
Statistical methods
All the data collected was compiled and formulated in Microsoft excel sheet and data was analyzed using SPSS version 20th software (Statistical package for social sciences).Descriptive statistics was done for all the variables. Data will be represented in the form of visual presentation i.e. pie diagram, bar diagram, etc. For qualitative data, Chi square test will be applied and quantitative data will be represented in the form of Mean and Standard deviation. Probability value (p 0.05) will be considered to be statistically significant.
Analysis of Ki-67
Regarding the analysis of Ki-67, The Ki-67 percentage scoring is defined as the percentage positivity of tumor cells stained among the total malignant cells assessed [13]. The histopathological specimen is checked for staining of the tumor cells. Scoring is performed upon the whole tumor section and not only limited to the hot spots of the cancer specimen.
IHC performed on the malignant cells staining positive for the Ki-67 is assessed in a quantitative way and by using a light microscope.
A Ki-67 cut-off point of 15 % was defined according to the experience of different pathologists as well as national and international recommendations at present [1,7,10,14,15].
Ki-67 values are obtained as the positive percentage in the malignant cells using MIB1 (anti-human Ki-67 monoclonal antibody) which is considered as the “gold standard” 16 .
Results
Study population
In our study duration, we had 250 cases of primary breast patients P.Total 50 patients were excluded from the primary data, as they did not meet the inclusion criteria. Thus, our sample size turned out to be 200 as due to time constraints. In our long study period, only one case of male carcinoma breast was reported. Minimum age of presentation being 25 years and maximum at 80 years with maximum number of females in the group of 41-60 years indicating highest risk of cancer breast in this age group. Further management depending upon histopathology report with ki-67and hormonal status.
Additionally, data including size (<3 vs. ≥3 cms), histological type and IDC Grade, nodal status, Progesterone Receptor (PR), ER, HER2, and Ki-67 were taken from the pathological reports. In particular, for what concerns Ki67, we set cut-points at, <20% (low) and >20% (high) as recommended by St. Gallen experts [1,17].
To create a comprehensive index associated to “good” and “poor” prognosis and based on our results, a comprehensive index or prognostic index was created similar to Cambridge Post Mastectomy Radiotherapy (C-PMRT) index. But, keeping our goal for chemotherapy rather than radiotherapy after the results.
Following the performance curves, we set the index cut-off at 3, indicating <3 a good prognosis and ≥3 a poor prognosis. Patients with an index ≥3 had a significant increased risk of relapse. Our total number with 85 out of 200 were at more risk thus, were subject to evaluation for additional hormone and chemotherapy therapy taking in account other parameters like ER/PR/HER-2.
The <20% and >20 % Ki67 cut-offs were correlated to IDC grades (P<0.0001), lymph node positivity (P<0.0001) and tumor size (P=0.007), ER + (P<0.0001). Patients with tumor with Ki67 > 20% showed the poorest prognosis.
It is also predictive of DSS and DFI, confirming that tumor burden and proliferative index which remains the most important parameter in breast cancer prognosis, as suggested by several other studies [18-22].
We also observed, Ki67 may be an important factor for the poor prognosis of TNBC. This indicated that the increased expression of Ki67 may predict the increased proliferation of breast cancer cells, enhanced Invasiveness, and faster growth of the tumor and the high incidence of lymph node metastasis. Therefore, expression of Ki67 might indicate poor prognosis in TNBC.
Thus, we concluded for low risk is <20% and high risk is >20%.
And keeping grade I and II in low risk and grade III of IDC in high risk we created a 2*2 box for specificity of ki -67.
On our collected data of 200 patients evaluated.
Table 5: The prognostic index was designed using the following parameter score values: (tumor size) + (number of metastatic lymph nodes) + (Ki67 score value), ranging from 0-5.
Table 6: We found that in 200 we had a final index of 0; 15 of 1; 45 of 2;55 of 3;43 of 4;33and 9 of 5.
Concluding
Higher values > 20% of ki67 we can almost predict 95% that the grade of the tumor is IDC grade III P<0.0001.
With almost 80 % accuracy that higher is the proliferating index with the higer value of ki-67.
Positive predictive value approximately 95%.
Specificity equals to 95.83% indicating that the test is not mandatory in lower grades (IDC I/II)
Discussion
In this study, we investigated the expression of Ki-67 and its relationship with other clinico-pathological parameters especially with the IDC grades and the expression of other molecular markers in 200 females of primary Breast Cancer patients (range: 25 to 80 years). Patients were from a single tertiary care centre and the clinical importance of Ki-67 as a prognostic marker of Breast Cancer was assessed. Of these ,72 were Ki-67 >20% using IHC , which correlated with poor prognostic features such as poor IDC grade , lymph nodal involvement, stage of the tumor and TNM stage had convincing value in identification of high risk women with Invasive ductal Breast Cancer.
It is a wise step and helps in
Discriminating patients at low or high risk of recurrence.
To correlate IDC grades with percentage positivity of ki-67.
The usefulness of the Ki67 proliferative index in breast cancer management is a matter of debate. A number of oncologists/oncosurgeons/breast surgeons do not rely on Ki67 in the clinical practice, due to its low reproducibility [17,23-30]. In addition, the National Comprehensive Cancer Network (NCCN) doesn’t impart any guidelines or information regarding the Ki67 assessment status and its importance in breast cancer prognosis [31]. However, the Saint Gallen Consensus Meeting suggests the usefulness of the Ki67 for stratifying Luminal cancers since 2009. Still, ambiguity regarding the definition of highly proliferating tumors remains. In 2011, a cut-off of 14 % 1was proposed, on the basis of the study by Cheang et al. [24], but 2 years later, it was upgraded to 20 %17. In the same year, Denkert et al. [32] proposed to considered Ki67 a continuum variable [33].
To solve these confusion, we evaluated Ki67 in a subset of patients with grades of IDC breast cancer, with the aim to corroborate its significance. Firstly, we determined the median value of Ki67 in our series, as suggested by the last Saint Gallen Consensus Meeting [34] and showed akin values of 14 % identified by Cheang [24] as able to differentiate Luminal A and B subtypes. As a sequel, when the motive is to distinguish high-risk patients,
the 20% cut-off is dependable than the 14% cut-off. Although, we confirm that Ki67 is a reliable marker for scrutinizing patients at risk for local and systemic recurrenceses and death, we believe that Ki67 percentage has to be evaluated following the International Guidelines [35]. The laboratory standardisation should be strictly subjected to verification of quality control and assessment [36]. Furthermore, similar to various other studies recommending that only one marker is not advocated for stratifying prognosis of breast cancer patients, we planned a comprehensive study index including Ki67 with tumor size and number of lymph nodes. As, both the tumor size and the number of axillary lymph nodes are conventionally taken as a prognostic marker in breast cancer patients [37,38].
Many Studies have also verified the value of Ki-67 expression to anticipant response to NACT. Chang et al. [39] and Archer et al. [40] reported a correlation between pretreatment Ki-67 labeling index and better response to chemotherapy in neoadjuvant settings. A 2005 review article [41] of five studies (with variable number of the patients ranging from 106 to 399) [42-46], that scrutinized the predictive value of the Ki-67 labeling index in the neoadjuvant setting; two of these studies [42,43] concluded that a high Ki-67 labeling index is associated with good response to Chemotherapy; however , the other studies [44-46] implied no similar association.
Cancer patients having complete clinical, radiological, histological, and follow-up data was Collected and compared. The Ki67 (<20% or >20%) cut-offs were correlated to IDC grades (P<0.0001), lymph node positivity (P<0.0001) and tumor size (P=0.007), ER + (P<0.0001). Patients with tumor with Ki67 >20% showed the poorest prognosis.
As per the new AJCC 8th staging system, which includes molecular biomarkers for staging of patients, it defines the prognosis of carcinoma of breast patients in better terms. AJCC 8th includes grading of tumors and here this study shows statistically significant correlation between Ki-67 with other parameters of AJCC 8th staging system as T, N, ER, PR, IDC grades. Additionally, it correlates well with the ki 67 and is a good tool for clinical decision making in limited resources.
Limitations
Of this study is that it included only patients of marathwada region; However, results may vary in other regions/countries. Additionally, other limitations like recurrence status of the patients could not be evaluated in every patient due to time constraints; thus, we further advise larger-scale multi centric studies for prognosticating significance of the ki67 in terms of tumor recurrence and disease-free survival.
Our data indicates that Ki-67 individually does not predict which patients would be benefitted more by addition of hormone therapy and chemotherapy in the adjuvant setting. It is just one of the significant contributing factors amongst other prognostic markers. Isolated ki-67 value does not have any recommendations for adding of hormone to regular chemotherapy regime. While, our data suggests that a high Ki-67 labeling index is significantly associated with other risk factors and can be divided on the bases of prognostic index.
Thus, in this study the Ki-67 index was used as a prognostic factor, not a predictive factor.
Conclusion
To conclude, we confirm the reliability of the Ki 67 as a prognostic marker in breast cancer patients, using a cut-off value of 20 %, and we stress the important role of Ki 67 in the clinical management and treatment planning of patients. It is easily correlated with grads of IDC explaining greater the value of ki-67 higher is the Modified Bloom Richardson’s score with greater tumor size and multiple lymph nodes involvement. However, waiting for molecular test accessibility, Ki 67 together with tumor size and lymph nodal status might be useful to identify breast cancer patients with poor prognostic outcome that may need combined chemotherapy and hormonal therapy. Additionally, it is implied by our results that in a non-affording patient with poor resources and financial constraints, who hails from a third world country we may omit prescribing ki-67 in grades I AND II IDC. Controversially, if the patient is affording even in the third world countries we should motivate the patients to get Ki-67 done for prognostication and correlation with the other markers of breast cancer. We can practically advocate that the use of prognostic index for breast cancer could “proxy” the multigene or other prognostic test results in third world countries.
References
- Goldhirsch A, Wood W, Coates A, Gelber R, Thurlimann B, et al. Strategies for subtypes dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011.Annals of Oncology. 2011; 22:1736-1747.
- HanahanDWeinberg R. The Hallmarks of Cancer. Cell. 2000; 100: 57-70.
- Milde-Langosch K, Karn T, Müller V, Witzel I, Rody A, et al. Validity of the proliferation markers Ki67, TOP2A, and RacGAP1 in molecular subgroups of breast cancer. Breast Cancer Res Treat. 2012; 137: 57-67.
- Wirapati P, Sotiriou C, Kunkel S, Farmer P, Pradervand S, et al. Meta-analysis of gene-expression profiles in breast cancer: toward a unified understanding of breast cancer sub-typing and prognosis signatures. Breast Cancersearch. 2008; 10: R65.
- Garcia RL, Coltrera MD, Gown AM. Analysis of proliferative grade using anti- PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. The American Journal of Pathology. 1989; 134: 733-739.
- IgnatiadisMSotiriou C. Understanding the Molecular Basis of Histologic Grade. Pathobiology. 2008; 75: 104-111.
- Stuart-Harris R, Caldas C, Pinder S, Pharoah P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. The Breast. 2008; 17: 323-334.
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol.1984; 133: 1710-1715.
- ScholzenTGerdes J. The Ki-67 protein: From the known and the unknown. J CellPhys. 2000; 182: 311-322.
- Yerushalmi R, Woods R, Ravdin P, Hayes M, Gelmon K. Ki67 in breast cancer: Prognostic and predictive potential. The Lancet Oncology. 2010; 11: 174-183.
- Luporsi E, André F, Spyratos F, Martin P, Jacquemier J, et al. Ki- 67: Level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2011; 132: 895-915.
- Statistics of Breast Cancer in India: Aurangabad. Breastcancerindia.net. 2016.
- Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J ClinOncol. 2005; 23: 7212-7220.
- Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, et al. 13th St. Gallen International Breast Cancer Conference 2013: Primary Therapy of Early Breast Cancer Evidence, Controversies, Consensus - Opinion of a German Team of Experts (Zurich 2013). Breast Care. 2013; 8: 1-1.
- De Azambuja E, Cardoso F, de Castro G, Colozza M, Mano M, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer. 2007; 96: 1504-1513.
- Dowsett M, Nielsen T, A’Hern R, Bartlett J, Coombes R, et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. JNCI Journal of the National Cancer Institute.2 011; 103: 1656-1664.
- Senn H. St. Gallen Consensus 2013: Optimizing and Personalizing Primary Curative Therapy of Breast Cancer Worldwide. Breast Care. 2013; 8: 101-101.
- Duffy S, Tabar L, Vitak B, Warwick J. Tumor Size and Breast Cancer Detection: What Might Be the Effect of a Less Sensitive Screening Tool Than Mammography?.The Breast Journal. 2006; 12: S91-S95.
- Verschraegen C, Vinh-Hung V, Cserni G, Gordon R, Royceet ME, et al. Modeling the Effect of Tumor Size in Early Breast Cancer. Annals of Surgery. 2005; 241: 309-318.
- Donegan W. Tumor-related prognostic factors for breast cancer. CA: A Cancer Journal for Clinicians. 1997; 47: 28-51.
- Danko M, Bennett K, Zhai J, Marks J, Olson J. Improved Staging in Node-Positive Breast Cancer Patients Using Lymph Node Ratio: Results in 1,788 Patients with Long-term Follow-Up. Journal of the American College of Surgeons. 2010; 210: 797-805.e1.
- De Azambuja E, Cardoso F, de Castro G, M Colozza, M S Mano, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. British Journal of Cancer. 2007; 96: 1504-1513.
- Gerdes J, Li L, Schlueter C, C Wohlenberg, C Gerlach, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. The American Journal of Pathology. 1991; 138: 867-873.73.
- Cheang MCU, Chia SK, Voduc D, Gao D, Leung S, et al. Ki67 Index, HER2 Status, and Prognosis of Patients With Luminal B Breast Cancer. JNCI Journal of the National Cancer Institute. 2009; 101: 736-750.
- Varga Z, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, et al. How Reliable Is Ki-67 Immunohistochemistry in Grade 2 Breast Carcinomas? A QA Study of the Swiss Working Group of Breast- and Gynecopathologists. van Diest P, ed. PLoS ONE. 2012; 7: e37379.
- Polley M-YC, Leung SCY, McShane LM, Gao D, Hugh JC, et al. An International Ki67 Reproducibility Study. JNCI Journal of the National Cancer Institute. 2013; 105: 1897-1906.
- Jonat W, Arnold N. Is the Ki-67 labelling index ready for clinical use. Annals of Oncology. 2011; 22: 500-502.
- Gudlaugsson E, Skaland I, Janssen E, Smaaland R, Shao Z, et al. Comparison of the effect of different techniques for measurement of Ki67 proliferation on reproducibility and prognosis prediction accuracy in breast cancer.Histopathology. 2012; 61: 1134-1144.
- Mikami Y, Ueno T, Yoshimura K, Tsuda H, Kurosumi M, et al. Interobserver concordance of Ki67 labeling index in breast cancer: Japan Breast Cancer Research Group Ki67 Ring Study. Cancer Science. 2013; 104: 1539-1543.
- Polley M, Leung S, Gao D, Mastropasqua M, Zabaglo L, et al. An international study to increase concordance in Ki67 scoring. Modern Pathology. 2015; 28: 778-786
- NCCN - Evidence-Based Cancer Guidelines, Oncology Drug Compendium, Oncology Continuing Medical Education. Nccn.org. 2018.
- Denkert C, Loibl S, Muller B, Eidtmann H, Schmitt W, et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Annals of Oncology. 2013; 24: 2786-2793.
- Denkert C, von Minckwitz G. Reply to Ki67 in breast cancer: a useful prognostic marker! Ann Oncol. 2014; 25: 542-543.
- Coates A, Winer E, Goldhirsch A, Gelber R, Gnant M, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Annals of Oncology. 2015; 26: 1533-1546.
- Dowsett M, Nielsen T, A’Hern R, Bartlett J, Coombes R, et al. Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. JNCI Journal of the National Cancer Institute. 2011; 103: 1656-1664.
- Nordi QC - Immunohistochemical Quality Control [Internet]. Nordiqc.org. 2018.
- Cianfrocca M. Prognostic and Predictive Factors in Early-Stage Breast Cancer. The Oncologist. 2004; 9: 606-616.
- Schneeweiss A, Chia S, Hickish T, V Harvey, A Eniu, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracyclinefree chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Annals of Oncology. 2013; 24: 2278-2284.
- Chang J, Ormerod M, Powles TJ, Allred DC, Ashley SE, et al. Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma, Cancer. 2000; 89: 2145-2152.
- Archer CD, Parton M, Smith IE, PA Ellis, J Salter, et al. Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer, Br J Cancer. 2003; 89: 1035-1041.
- Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, et al. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now?, Ann Oncol. 2005; 16: 1723-1739.
- MacGrogan G, Mauriac L, Durand M, F Bonichon, M Trojani, et al. Primary chemotherapy in breast Invasive carcinoma: predictive value of the immunohistochemical detection of hormonal receptors, p53, c-erbB-2, MiB1, pS2 and GST pi, Br J Cancer. 1996; 74: 1458-1465.
- Assersohn L, Salter J, Powles TJ, R A’hern, A Makris, et al. Studies of the potential utility of Ki67 as a predictive molecular marker of clinical response in primary breast cancer, Breast Cancer Res Treat. 2003; 82: 113-123.
- Bottini A, Berruti A, Bersiga A, MP Brizzi, P Bruzzi, et al. Relationship between tumour shrinkage and reduction in Ki67 expression after primary chemotherapy in human breast cancer, Br J Cancer. 2001; 85: 1106-1112.
- Colleoni M, Zahrieh D, Gelber RD, A Luini, V Galimberti, et al. Preoperative systemic treatment: Prediction of responsiveness, Breast. 2003; 12: 538-542.
- Chang J, Powles TJ, Allred DC, SE Ashley, GM Clark, et al. Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer, J Clin Oncol. 1999; 17: 3058-3063.