- Research Article
- |
- Open Access
Predictors of complete response to neoadjuvant chemotherapy in Breast Cancer: A National Cancer Database (NCDB) analysis
- Marissa K Srour;
- Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, California, USA
- Jeffrey Johnson;
- Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, California, USA
- Alice Chung;
- Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, California, USA
- Armando E Giuliano;
- Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, California, USA
- Farin Amersi
- Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, California, USA

| Received | : | Dec 30, 2019 |
| Accepted | : | Feb 18, 2020 |
| Published Online | : | Feb 24, 2020 |
| Journal | : | Annals of Breast Cancer |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Srour MK, Johnson J, Chung A, Giuliano AE, Amersi F. Predictors of complete response to neoadjuvant chemotherapy in Breast Cancer: A National Cancer Database (NCDB) analysis. Ann Breast Cancer. 2020; 3(1): 1013.
Abstract
Purpose: Pathologic Complete Response (PCR) following Neoadjuvant chemotherapy (NAC) for breast cancer has been associated with improved survival. The objective was to characterize those patients receiving NAC and analyze predictors of PCR.
Patients and methods:
Patients with clinical stage I-IIIC breast cancer who received NAC from 2004-2011 were identified from the National Cancer Database (NCDB). Multivariable analysis identified factors predicting PCR after NAC.
Results:
Of 49,850 patients, mean age was 53 (SD±13 years). Ductal carcinoma comprised 78% of tumors. Clinical stage I, II, and III were represented in 11%, 52%, and 37% of patients, respectively. Estrogen receptor was expressed in 60% of tumors (ER+). HER2 overexpression (HER2+) was seen in 8% of tumors and 7% of patients had triple negative disease (ER-/PR-/HER2-). Multivariable analysis was performed among 13,825 women with PCR data to identify significant predictors of response to NAC. Strong predictors of PCR (OR>1.5) included absence of lymphovascular invasion (LVI), early-stage disease, and ER-/PR- status, whereas well and moderately differentiated tumors strongly predicted (OR<0.67) partial or no PCR.
Conclusion:
In a large cohort of women with breast cancer treated with NAC, PCR is most often seen in patients with hormone receptor negative, early clinical stage disease without LVI.Introduction
Neoadjuvant Chemotherapy (NAC) for breast cancer is used to downstage locally advanced disease to improve candidacy for breast conserving surgery (BCS)[1]. Although several studies have shown no survival benefit of NAC compared to adjuvant chemotherapy [1-6], achieving a pathologic complete response (PCR) following NAC has been associated with improved survival [1,2]. PCR has been proposed as a surrogate endpoint for prediction of long-term clinical benefit, including disease-free survival and overall survival [1-3]. While no studies have validated PCR as an endpoint for survival, the association between PCR and long-term outcomes is strongest in patients with triplenegative and HER2-positive breast cancer [1].
Patients are selected for NAC based on tumor histology, size, receptor subtype, nodal involvement, and patient age/co-morbidities. There is a paucity of data in the literature identifying which clinicopathologic factors may predict the effectiveness of NAC. A recent meta-analysis of 10 randomized clinical trials comparing NAC to the same chemotherapy given postoperatively, showed that although there is no survival difference between the groups, patients who had NAC had higher rates of loco-regional recurrence following breast conserving surgery [1]. In order to select patients who may benefit from NAC as opposed to adjuvant chemotherapy, it is important to better predict which patients will have a good response to NAC. The purpose of this study was to characterize patients receiving NAC and analyze predictors of PCR. Specifically, we examined clinicopathologic variables associated with PCR.
Methods
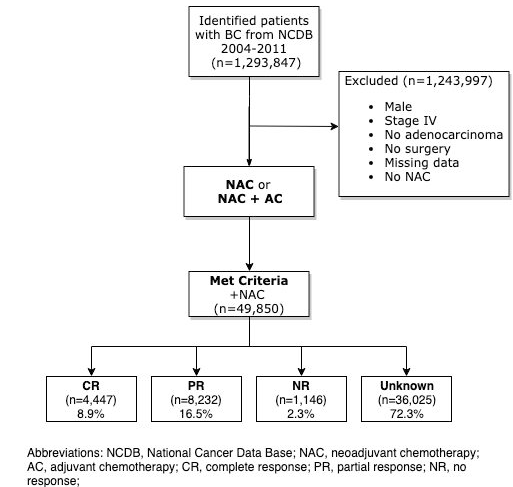
Patients from the National Cancer Database (NCDB) who had clinical stage I-IIIC invasive breast cancer and received NAC from 2004 to 2011 were identified. Patients who were male, had stage IV disease, had no invasive ductal or lobular disease, no surgery, or missing data were excluded from analysis. Patients who had NAC or NAC plus adjuvant chemotherapy (AC) were included in the analysis. Patients were divided into four groups based on response to NAC: Complete Response (CR), Partial Response (PR), No Response (NR), or unknown.
Patient variables collected included year of diagnosis, age, race, histology, clinical stage, hormone receptor status, grade, and lymphovascular invasion. The primary outcome measure was clinical and pathological characteristics associated with PCR.
Data are presented as frequency (percentage, %) for categorical variables and mean (±standard deviation) for numerical variables. Univariate associations between variables were examined with Kruskal-Wallis test, chi-square test or Fisher’s exact test, where appropriate. Post-hoc pairwise comparisons using the Bonferroni correction adjusting for inflation due to multiple comparisons were further performed where significant associations (p-value < 0.05) were found. Cox multivariate regression was performed to identify those factors predicting PCR after NAC. All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina) and R package version 3.4.1 with two-sided tests and a significance level of 0.05.
Results
49,850 women with clinical stage IA through IIIC invasive ductal carcinoma (IDC) or invasive lobular carcinoma (ILC) who had NAC with subsequent surgery and met inclusion criteria were identified (Figure 1). Of these 49,850 patients who had NAC, mean age was 53 years (SD ± 13 years). 77.3% of patients were white, and 17.2% were black. 77.6% of patients had IDC and 7.4% had ILC. Most patients had clinical stage II cancer (52.2%), followed by stage III (37.0%) disease. Estrogen receptor was expressed in 59.7% of tumors (ER+), and 78% of ER+ tumors also expressed progesterone receptor (PR+). More specifically, 46.8% of patients were ER/PR positive, 35.7% ER/PR negative, and 6.9% of patients had triple-negative breast cancer. 73.5% of patients did not have reported HER2 data. For the 13,231 patients with available HER2 data, 7.6% were HER2 positive, and 19.0% were HER2 negative. The majority of patients had high grade disease (50.4%) (Table 1).
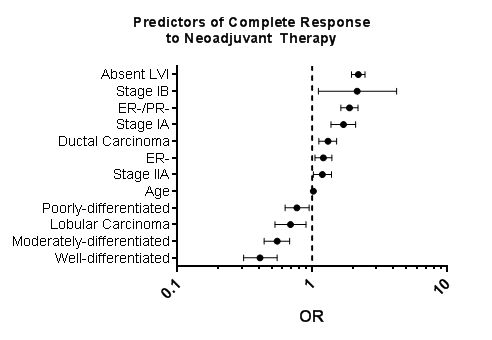
Of the 49,850 patients who had NAC, 4,447 (8.9%) patients had a CR, 8,232 (16.5%) patients had a PR, 1,146 (2.3%) patients had NR, and 36,025 (72.3%) patients had unknown response (Figure 1). Of the 13,825 women with PCR data, multivariable analysis was performed to identify significant predictors of response to NAC. Insurance status and year of treatment were not statistically significant and were excluded from the analysis. HER2+ status was excluded due to missing data. Strong predictors of PCR (OR>1.5) included absence of lymphovascular invasion (LVI) (OR 2.20, 95% CI 2.97, 2.47; p<0.001), early stage disease (Stage IB: OR 2.16, 95% CI 1.11, 4.24, p=0.024; Stage IA: OR1.71, 95% CI 1.38, 2.10, p<0.001), and ER- status, (ER-/PR-: OR 1.89, 95% CI 1.63, 2.19, p<0.001; ER-: OR 1.21, 95% CI 1.05, 1.40, p=0.009) (Figure 2) (Table 2).
Conversely, well (OR 0.41, 95% CI 0.31, 0.55; p<0.001) and moderately (OR 0.55, 95% CI 0.44, 0.68; p<0.001) differentiated grade strongly predicted (OR<0.57) partial or NR. Ductal carcinoma was associated with PCR (OR 1.31, 95% CI 1.12, 1.52; p=0.001), however lobular carcinoma was associated with a partial or NR (OR 0.69, 95% CI 0.53, 0.90; p=0.007) (Figure 2) (Table 2).
Discussion
To our knowledge, this is the largest study examining predictors of PCR among women with breast cancer treated with NAC. NAC was historically used to downstage locally advanced cancer to make inoperable tumors operable [1]. Subsequently, NAC was used to reduce tumor burden to allow for breast conserving therapy [1]. While several studies have shown no difference in survival in NAC compared to AC [1-3,7,17], patients who achieve PCR following NAC may have improved survival [1,2].
Single institution studies have shown excellent response rates for women with breast cancer following NAC [19]. Symmans et al. examined 381 women with BC who had NAC and reported patients with minimal residual disease carried the same prognosis as patients with PCR, while those with extensive residual disease had a poor prognosis1 . Liedtke et al. examined 255 patients with triple negative breast cancer (TNBC) who received NAC and showed a higher PCR rate, but lower 3-year diseasefree (DFS) and overall (OS) survival compared to patients with non-TNBC [2]. These studies demonstrated the importance of PCR on survival rates, but they did not look at predictors of PCR. Chaudry et al. evaluated 749 women with BC who achieved PCR after NAC and showed patients over the age of 50 or with clinical stage IIIB or IIIC had increased risk of distant metastasis and lower overall survival [12]. While our study did not examine survival, as survival data is not available in the NCDB for analysis, our study similarly showed that lower clinical stage (IB and IIA) was a predictor of PCR following NAC. Lower stage may be predictive of PCR due to a decreased tumor volume necessary to respond to chemotherapy drugs in order to achieve a complete resolution of tumor cells.
Several large-scale studies using national databases have evaluated patients with breast cancer who had PCR. Barron et al. identified 30,281 patients with cT1-2 BC who received NAC from the NCDB between 2010 to 2015 and showed that 13% of patients with hormone-receptor positive, HER2 negative BC, 37% of patients with triple positive BC, 37% with TNBC, and 58% with HER2 positive BC achieved PCR [2]. While this study defined the rates of PCR in both node-positive and node-negative disease, they did not analyze predictors for PCR. Puig et al. identified 171,985 patients with ER negative breast cancer from the NCDB between 2004-2012 and found the overall rate of PCR was 23.9% in the breast, 58.4% in the in the axilla, and 21.0% in both the breast and axilla [2]. The authors found a significant relationship between clinical N stage and axillary PCR, where axillary PCR was found in 86.1% of patients with clinically negative nodes, and 43.7% of patients with clinically positive nodes (p<0.001). Haque et al. examined 13,939 women from the NCDB between 2004 to 2014 with BC who had PCR and showed that molecular subtype was an independent predictor of both PCR, which was lowest in luminal A (0.3%) and highest in HER2 positive tumors (38.7%) [2]. This study performed a multivariable logistic regression analysis for factors predictive of PCR, and found luminal B, HER2 positive, and triple negative BC subtypes to be predictive of PCR. Our study similarly found TNBC to be a predictor of PCR, but our study did not evaluate HER2 receptor subtype due to the lack of reliable data. They similarly found increased PCR for lower clinical stage. However, their study did not evaluate for histology of ductal versus lobular carcinoma, grade, LVI, or other clinical and pathological characteristics.
A limitation to the NCDB is there was only survival data available for breast cancer from 2004-2006, and survival related to PCR could not be analyzed. Additionally, HER2 data was excluded from analysis of predictors of NAC due to missing data.
Conclusion
In a large cohort of women with breast cancer treated with NAC, PCR is most often seen in patients with hormone receptor negative, early clinical stage disease without LVI. Patients with these tumor characteristics should be strongly considered for NAC. Patients with well-differentiated tumors and those with lymphatic involvement are less likely to benefit.
Acknowledgements
The Fashion Footwear Charitable Foundation of New York, Inc.; The Margie and Robert E. Petersen Foundation; Associates for Breast and Prostate Cancer Studies; Linda and Jim Lippman.
References
- Clough KB, Acosta-Marin V, Nos C, Alran S, Rouanet P, et al. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: a French national survey. Annals of Surgical Oncology. 2015; 22: 3504-3511.
- Ragaz J, Baird R, Rebbeck P, et al. Preoperative (neoadjuvant) versus postoperative adjuvant chemotherapy for stage I–II breast cancer. Long-term analysis of British Columbia randomized trial. Proc Am Soc Clin Oncol. 1997; 16: 142a.
- Gazet JC, Ford HT, Gray R, McConkey C, Sutcliffe R, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Annals of Oncology. 2001; 12: 685-691.
- Danforth DN Jr, Cowan K, Altemus R, Merino M, Chow C, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Ann Surg Oncol. 2003; 10: 635-644.
- Taucher S, Steger GG, Jakesz R, Tausch C, Wette V, et al. The potential risk of neoadjuvant chemotherapy in breast cancer patients-results from a prospective randomized trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-07). Breast Cancer Res Treat. 2008; 112: 309-316.
- Powles TJ, Hickish TF, Makris A, Ashley SE, O’Brien ME, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol. 1995; 13: 547-552.
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. Journal of the National Cancer Institute Monographs. 2001; 30: 96-102.
- Vander Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19: 4224-4237.
- Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicentre randomized trial with a 124-month median follow-up. Ann Oncol. 1999; 10: 47-52.
- Scholl SM, Asselain B, Palangie T, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991; 27: 1668- 1671.
- Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017; 15: 1216-1223.
- Chaudry M, Lei X, Gonzalez-Angulo, Mittendorf EA, valero V, et al. Recurrence and survival among breast cancer patients achieveing a pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015; 153: 417-423.
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L. Preoperative chemotherapy in operable breast cancer. Lancet. 1993; 341: 1485.
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L. Preoperative chemotherapy in operable breast cancer. Lancet. 1993; 341: 1485.
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007; 2: CD005002.
- Cortazar P, Zhang L, Untch M. Mehta K, Costantino JP, et al. Pathologic complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. The Lancet. 2014; 384: 164-172.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomized trials. Lancet Oncology. 19: 27- 39.
- Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer. 1980; 16: 351-356.
- Cook M, Johnson N. Pre-surgical chemotherapy for breast cancer may be associated with improved outcomes. The American Journal of Surgery. 2018; 215: 931-934.
- Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, et al. A. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009; 27: 2474- 2481.
- Mauri D, Pavlidis N, Loannidis JP. Neoadjuvant versus adjuavant systemic treatment in breast cancer: a meta-analysis. Journal of the National Cancer Institute. 2005; 97: 188-194.
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. Journal of Clinical Oncology. 2012; 30: 1796-1804.
- Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. Journal of the National Cancer Institute. 2005; 97: 1137-1142.
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, et al. Measurements of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. Journal of Clinical Oncology. 2007; 25: 4414-4422.
- Liedtke C, Mazouni C, Hess KR. Response to neoadjuvant therpay and long-term survival in patients with triple-negative breast cancer. Journal of Clinical Oncology. 2008; 26: 1275-1281.
- Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surgery. 2018; 153: 1120-1126.
- Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a national cancer data base study. Annals of Surgical Oncology. 2017; 24: 1242-1250.
- Haque W, Verma V, Hatch S, Klimberg VS, Butler EB, et al. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Research and Treatment. 2018; 170: 559-567.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.