Annals of Bariatrics & Metabolic Surgery
HOME /JOURNALS/Annals of Bariatrics & Metabolic Surgery- Research Article
- |
- Open Access
Maximum preoperative weight loss as a predictor of weight loss surgery outcomes
- Caroline Park;
- Department of General Surgery/Minimally Invasive Surgery, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
- George L Blackburn;
- Department of General Surgery/Minimally Invasive Surgery, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
- Daniel B Jones;
- Department of General Surgery/Minimally Invasive Surgery, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
- Hussna Wakily;
- Department of General Surgery/Minimally Invasive Surgery, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA
- Benjamin E Schneider
- Department of General Surgery/Minimally Invasive Surgery, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA

| Received | : | Apr 18, 2018 |
| Accepted | : | Oct 10, 2018 |
| Published Online | : | Oct 18, 2018 |
| Journal | : | Annals of Bariatrics & Metabolic Surgery |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Park C, Blackburn GL, Jones DB, Wakily H, Schneider BE. Maximum preoperative weight loss as a Predictor of Weight Loss Surgery Outcomes. Ann Bariatr Metab Surg. 2018; 1: 1004.
Abstract
Background: Maximum Preoperative Weight Loss (MPWL) is defined as the maximum amount of weight lost in a patient’s lifetime prior to Weight Loss Surgery (WLS). We hypothesized that higher MPWL would predict greater postoperative weight loss in patients who underwent WLS.
Methods: This is a single-center retrospective study of patients who underwent WLS from 2004-2012. Outcomes were compared at 3, 6, and 12 months. Pearson’s coefficient r was used to evaluate the relationship between MPWL and total weight loss with p value of <0.05. Maximum pre-operative weight loss was divided into categories of 0-40lb., 41- 80lb., 8l-280lb. in order to create categories appropriate for a logistic regression. Logistic regression was used to identify whether MPWL predicted postoperative weight loss outcomes. Multiple regression was used to identify a relationship between MPWL, age, gender, type of procedure, and postoperative variables of weight loss and BMI at 3, 6 and 12 months.
Results: The study included a total of 506 patients (mean age 44.9 years; 75% female). Mean self-reported weight loss across all procedures was 47.9 lbs; mean BMI was 46.7 kg/m2 . Whole group analysis found a positive correlation between MPWL and total weight loss (P < 0.001). Subgroup analyses by procedure showed a positive correlation between BMI and MPWL at 12 months (P = 0.015).
Conclusions: maximum preoperative weight loss may be a significant predictor of total weight loss at 1 year for patients who undergo weight loss surgery.
Keywords: Park C, Blackburn GL, Jones DB, Wakily H, Schneider BE. Maximum preoperative weight loss as a Predictor of Weight Loss Surgery Outcomes. Ann Bariatr Metab Surg. 2018; 1: 1004.
Background
Obesity continues to be a growing epidemic worldwide [1], one that imposes a considerable economic burden on society [2]. More than a decade ago, The National Institutes of Health (NIH) [3] reported that individuals affected by severe obesity are resistant to maintaining weight loss achieved by conventional therapies (e.g., consuming fewer calories, increasing exercise, commercial weightloss programs) [4]. Scheen et al. Approached the complexity of obesity, its physiology and relationship with insulin resistance and the emphasized the importance of a multi-system, multi-disciplinary approach to combating obesity and diabetes [5]. Since that time, weight loss surgery has become the most effective and durable treatment for obesity and its comorbidities [1,6].
Most studies demonstrate that more than 90% of individuals previously affected by severe obesity are successful in maintaining 50% or more of their excess weight loss following bariatric surgery; among those affected by super severe obesity, more than 80%are able to maintain more than 50% excess body weight loss [4]. With the advent of laparoscopy, mortality rates are now under 1 per 1400 cases in accredited centers [7].
Although many studies have been carried out on the effects of preoperative weight loss on long-term outcomes of various surgical procedures, results have been inconsistent. To our knowledge, no research has been done on the impact of Maximum Preoperative Weight Loss (MPWL), or weight lost in a patient’s lifetime prior to weight loss surgery.
This study included long-term outcomes of Roux-en-Y gastric bypass (laparoscopic or open), laparoscopic adjustable gastric banding, and laparoscopic sleeve gastrectomy. To address whether MPWL has an effect on long-term weight loss across this range of surgical procedures, we designed a retrospective study that measured MPWL, with postoperative follow-up at 3, 6, and 12 months. Our primary hypothesis was that the higher the MPWL, the greater the long-term weight loss.
Methods
Subjects
Men and women between the ages of 19 and 70 years who met NIH consensus criteria [9] for weight loss surgery (BMI > 40 kg/m2 or BMI of 35-40 kg/m2 with high-risk obesity-related comorbidities) were included in the study. Each had undergone laparoscopic band, sleeve gastrectomy, or Roux-en-Y gastric bypass between 2004-2012 at an academic medical center. Each provided a self-report of MPWL, which is defined as the maximum amount of intentional weight loss achieved using a variety of diets, exercise, medications and other regimen. The period of attempted weight loss spanned adolescence to the time of preoperative evaluation. Data was self-reported and collected via pre-operative interview by the surgeon. Other intake variables included ethnicity, other comorbidities, such as hypertension, coronary artery disease, pulmonary disease (such as asthma, obstructive sleep apnea), history of smoking or alcohol use, and diabetes mellitus. Patients who had previous weight loss surgery were also included, though this group consisted of a small minority of patients (less than 1% of the cohort). Exclusion criteria included patients whose pre-operative evaluation did not include “maximum preoperative weight loss.” Female subjects used adequate contraception for the duration of the study. Pre-operative weight loss target of 10-15% was encouraged but not mandatory for patients; no specific pre-operative weight loss protocol was enforced, i.e. very-low calorie diet or other restrictive diets. This immediate pre-operative weightloss during evaluation was not included as part of ‘maximum preoperative weight loss’.
Methods
The IRB for this study is also referred as the ‘Committee on Clinical Investigation’ and is based in the Beth Israel Deaconess Medical Center, Boston, MA, 02215. Informed consent to participate in the study was obtained as part of the patient’s initial intake and written surgical consent.
We removed all potential identifying information, and assigned each patient a unique identifier. Each patient underwent a preoperative workup that included the collection of data on MPWL, BMI, weight (lbs.), gender, and prior history of weight loss surgery. All patients were evaluated by a nutritionist and a surgeon at 3, 6, and 12 months. Data collected at each standardized postoperative visit included current BMI, weight (lbs.), excess weight loss and percentage of excess weight loss, and total weight loss and percentage of total weight loss. Specific calculations for excess weight loss and percentage of excess weight loss are as follows and were recorded but not included in our final data analysis:
Excess weight loss (lbs) = Current weight – Ideal body weight.
Percentage of excess weight loss = [(Initial Weight) – (Postoperative weight)]/[(Initial Weight)-(Ideal body weight)]
Statistical analysis
All analyses are for patients completing the full 12-month study; 3-month and 6-month analyses were included for those that did not complete the full 12-month study (139 patients or approximately 27% over the span of the 8 years of collected data). We used STATA® (College Station, Texas USA) to run a series of tests that included Pearson’s coefficient r to evaluate the relationship between maximum preoperative weight loss and total weight loss with p value of <0.05. Maximum pre-operative weight loss was divided into categories of 0-40lb., 41-80lb. up to 280lb in order to create categories appropriate for a logistic regression. Logistic regression was used to identify whether maximum preoperative weight loss predicted postoperative weight loss outcomes. Multiple regression was used to identify a relationship between maximum preoperative weight loss, age, gender, type of procedure, BMI and postoperative total weight loss, excess weight loss, and post-operative BMI at 3, 6 and 12 months.
Patients were split into surgical groups based on the respective procedure, which was determined by a combination of surgeon recommendation, patient preference and insurance approval. They then underwent laparoscopic banding, laparoscopic sleeve gastrectomy, or Roux-en-Y gastric bypass (open or laparoscopic) procedures. All procedures were performed by one surgeon with generalized standard bypass length of 150cm for a Roux-en-Y gastric bypass; the majority of procedures were performed laparoscopically.
Results
Preoperative
A total of 506 individuals met NIH criteria [9] for weight loss surgery. Their mean age was 44.9 years; mean BMI was 46.7 kg/m2 . Approximately 75% of patients were female. Minimum preoperative BMI ranged from 34.3 kg/m2 to 80.7 kg/m2 . All patients received a nutritional, psychosocial, and medical screening prior to surgical evaluation by a board-certified weight loss surgeon. Laparoscopic band procedures numbered 309 (61.0%), followed by 181 (35.8%) for Roux-en-Y gastric bypasses, and 16 (3.2%) for laparoscopic sleeve gastrectomies. Minimum MPWL ranged from 0 to 270 lbs. Mean MPWL was approximately 49 lbs (Table 1).
Postoperative
Mean MPWL for patients who underwent laparoscopic gastric band placement was 45 lbs. It was 56.2 lbs. for those who had Roux-en-Y gastric bypass, and 49.3 lbs. for those who had laparoscopic sleeve gastrectomies.
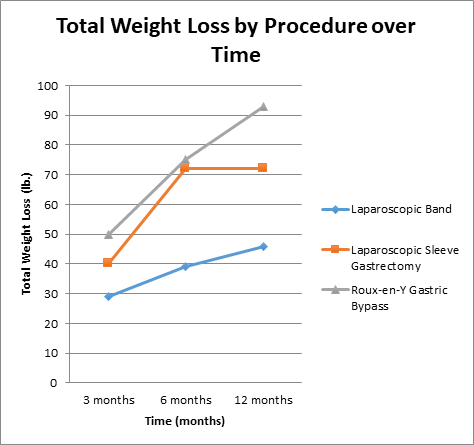
Patients who underwent laparoscopic gastric banding had less postoperative weight loss at all follow-up intervals, with a mean 12-month weight loss of 46.4 lbs. Those who had laparoscopic sleeve gastrectomy achieved weight loss of approximately 70 lbs. at the 6-month follow-up, with a mean weight loss of 71.7 lbs. at 12 months. Roux-en-Y gastric bypass produced the greatest amount of weight loss at all follow-up intervals, with a mean 12-month weight loss of 92.9 lbs (Figure 1a).
Figure 1a: Total post-operative weight loss (lbs.) by procedure over time (3 months, 6 months, 12 month intervals).
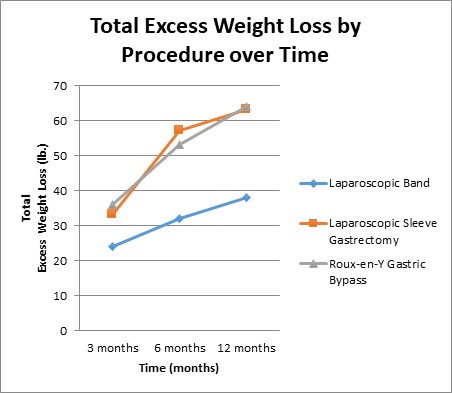
Total excess weight loss was lowest for laparoscopic banding patients; 38 lbs. at 12 months. At 1-year post-surgery, total excess weight loss for those who underwent laparoscopic sleeve gastrectomy and Roux-en-y gastric bypass were almost the same, with respective figures of 63 lbs. and 64 lbs (Figure 1b).
Figure 1b: Total post-operative excess weight loss (lbs.) by procedure over time (3 months, 6 months, 12 months).
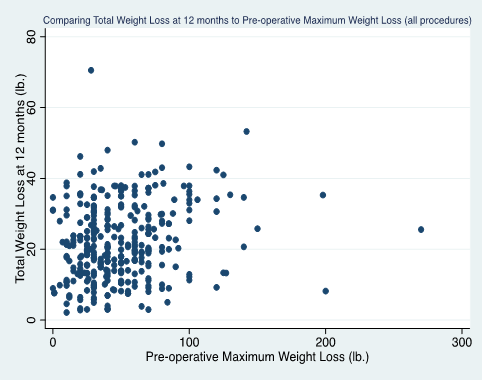
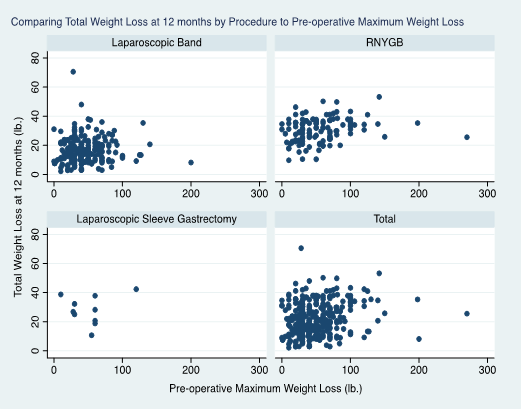
Across all procedures and follow-up intervals, MPWL was positively associated with postoperative total weight loss (see Figures 2a, 2b). Whole group analysis identified a positive correlation between MPWL and 12-month total weight loss (P < 0.011, r=0.10). Subgroup analyses by procedure showed a positive correlation between MPWL and 12-month total weight loss (for RNYGB, P < 0.015, r=0.21). No specific amount of MPWL (which was grouped in categories of 40 lbs. intervals (0-40 lbs., 41-80 lbs. up to 280lb.), was a significant predictor of postoperative weight loss between specific surgical groups or for all procedures as a whole.
Figure 2a: Total post-operative weight loss (lbs.) at 12 months as a function of pre-operative maximum weight loss (all procedures), p<0.011, r=0.10.
Discussion
In this study, MPWL was a significant predictor of postoperative total weight loss for all surgical procedures and follow-up periods. Along with BMI, it was also a significant positive predictor of 12-month weight loss across the full cohort of patients who underwent Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, or laparoscopic adjustable banding.
Many predictive measures of weight loss after various bariatric surgeries have been studied, including preoperative BMI and Health-Related Quality of Life (HRQOL) scores [12], timing of laparoscopic gastric bypass or sleeve gastrectomy [13], preoperative weight loss immediately before surgery [14,15], and other variables ranging from preoperative gut hormone responses to a standard meal before Roux-en-Y gastric bypass [16] to pre-surgical cortical activation to food pictures [17].
Saboor et al [12]. found that QOL scores prior to laparoscopic gastric banding did not have predictive value for excess weight loss 1 year out, but BMI had a significant, positive, and independent association. Ortega et al [13]. showed that prompt early referral for bariatric surgery was associated with greater weight loss. Livhits et al [14]. and Gerber et al [18]. reported that preoperative weight loss appeared to be associated with greater postoperative weight loss.
Repeated measures analysis showed that black, mixed, and missing races (combined) in comparison with the white race were associated with a decreased percent weight loss and less likelihood of a higher and sustained percent weight loss; and less obese patients were less likely to have higher and sustained percent weight loss after Roux-en-y gastric bypass surgery [19]. Recently, Manning et al [20] identified early postoperative weight loss as a predictor of maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass.
In perhaps the largest trial to date, Benoit et al [21]. used the Bariatric Outcomes Longitudinal Database (BOLD) to determine the contributions of various predictors to the large variations in absolute weight loss and percent BMI loss after bariatric surgery. They found that the key predictors for absolute weight loss at 12 months were procedure (44.8%) and baseline weight (18.5%), with 32.4% of the variability unexplained. Other significant predictors (i.e., age, race, diabetes) accounted for <1% of variability.
These findings indicate that research on additional sources of variability is still needed to help explain the remaining differences in outcomes after weight loss surgery [21]; that further studies are necessary to investigate whether preoperative factors can predict a clinically meaningful difference in weight loss after bariatric surgery [22]. The identification of predictive factors may improve patient selection and help develop interventions that target the specific needs of patients [22].
This study has a large number of limitations, including small sample size and uneven distribution of patients among the various surgeries. The sample includes a greater number of women than men, so it is uncertain whether results can be applied to men given the physiologic changes of peri-partum weight gain and loss in females. Pregnancy weight loss and gain was not accounted for in the initial intake and could have certainly influenced maximum pre-operative weight loss. Since patients were all treated at a single academic medical center, outcomes may not be generalizable to other environments. Follow-up of weight loss surgery patients may be hampered by the collection of data solely through visits with the patient’s bariatric surgeon, and the associated loss of follow-up among patients with treatment failure is a potential source of unmeasured bias in the analysis of long-term studies [23,24]. The length of the study at one year limits the evaluation of more long-term weight loss, which ideally would be measured at 5 to 10 years. In addition, self-report data can be highly inaccurate [25] and can be influenced by several factors as pregnancy, history of malignancy and administration of chemotherapy, etc.. These alternate factors should be considered and were not extensively evaluated in the pre-operative survey. We would also like to note that there are extensive physiologic differences and variability in weight loss for a 25 year-old versus 60 year-old patient, and that this area, under powered in this study, is of great interest as our populations continue to age with maintenance of multiple comorbidities that can affect weight loss outcomes.
We included patients who underwent laparoscopic sleeve gastrectomies; this group contributed to a small percentage of the overall cohort of patients (<5% at the time of this study in early 2010), but we felt that given the increasing popularity and success with sleeve gastrectomy at this point, we opted to include this cohort as it has eventually become one of the most prevalent weight loss procedures within the group practice and across the United States.
Lastly, we also included patients who had previous weight loss surgery though this group consisted of a small minority of patients (less than 1% of the cohort). We felt that because they contributed to such a small portion of the total patient population, it would not significantly affect our results, though we did consider this as a potential outlier. It is highly probable that the maximum preoperative weight loss of these patients was augmented by their first weight loss surgery with eventual regain within a few years. Besides the other factors that contribute to weight regain (pouch size, changes to high-calorie, low volume foods), these patients are also interesting in their biology and psychosocial adaptations to weight loss surgery. These patients remain a small minority but their struggles with maintaining weight loss despite maximal measures point towards a complex problem that requires a multi-disciplinary approach.
Conclusions
Overall, this study suggests that MPWL is a significant predictor of 12-month weight loss outcomes after Roux-en-Y gastric bypass, laparoscopic gastric banding, and laparoscopic sleeve gastrectomy. This information can serve as a springboard to future studies on the clinical utility of MPWL as a predictive outcome variable.
Declarations
Ethics approval and consent to participate: The IRB for this study is based in the Beth Israel Deaconess Medical Center, Boston, MA, 02215. This study was a retrospective review that did not include patient identifiers, presented no more than minimal risk (including physical and psychological) to its research subjects.
Consent for publication and availability of data and material: The data was collected via non-invasive procedures routinely employed in clinical practice (i.e. collection of data did not involve general anesthesia or sedation). Data was also previously collected for research purposes other than the currently proposed research. For the above reasons, neither additional clinical trial registration nor explicit written informed consent was required.
Author Contributions
CP, GB and DBJ conceived the research. CP coordinated the study design, generation of figures, data analysis and data interpretation with assistance for the latter by SG. Both CP and HW were involved with the data collection. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Acknowledgments
The authors thank Rita Buckley for editorial services provided in the development of this manuscript and Shiva Gautam, PhD of the Biostatistics Department at Harvard Medical School for his consultation.
References
- Chang J, Brethauer S. Medical Devices in the Treatment of Obesity. Endocrinol Metab Clin North Am. 2016; 45: 657-665.
- Arabi Basharic F, Olyaee Manesh A, Ranjbar Ezzat Abadi M, Shiryazdi SM, Shabahang H, Jangjoo A. Evaluation of laparoscopic sleeve gastrectomy compared with laparoscopic Rouxen-Y gastric bypass for people with morbid obesity: A systematic review and meta-analysis. Med J Islam Repub Iran. 2016: 354.
- NIH, National Institutes of Health, U.S. Department of Health and Human Services. Turning Discovery into Health. 2016.
- ASMBS, American Society for Metabolic and Bariatric Surgery. Benefits of Bariatric Surgery. 2016.
- Scheen AJ, Van Gaal LF. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014; 2: 911-922.
- Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr, et al. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016 ; 151: 1046-1055.
- Azagury DE, Morton JM. Bariatric Surgery: Overview of Procedures and Outcomes. Endocrinol Metab Clin North Am. 2016; 45: 647-656.
- Parri A, Benaiges D, Schroder H, Izquierdo-Pulido M, Ramón J, et al. Preoperative predictors of weight loss at 4 years following bariatric surgery. Nutr Clin Pract. 2015; 30: 420-424.
- Sethi M, Beitner M, Magrath M, Schwack B, Kurian M, et al. Previous weight loss as a predictor of weight loss outcomes after laparoscopic adjustable gastric banding. Surg Endosc. 2016; 30: 1771-1777.
- Pekkarinen T, Mustonen H, Sane T, Jaser N, Juuti A, et al. LongTerm Effect of Gastric Bypass and Sleeve Gastrectomy on Severe Obesity: Do Preoperative Weight Loss and Binge Eating Behavior Predict the Outcome of Bariatric Surgery? Obes Surg. 2016; 26: 2161-2167.
- NIH Consensus Development Conference, March 25-27, 1991. Gastrointestinal Surgery for Severe Obesity. 1991; 9: 25-27.
- Saboor Aftab SA, Halder L, Piya MK, Reddy N, Fraser I, et al. Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg. 2014; 24: 885-890.
- Ortega E, Morinigo R, Flores L, Moize V, Rios M, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012; 26: 1744-1750.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, et al. Does weight loss immediately before bariatric surgery improve outcomes: a systematic review. Surg Obes Relat Dis. 2009; 5: 713- 721.
- Brown WA, Moszkowicz J, Brennan L, Burton PR, Anderson M, et al. Pre-operative weight loss does not predict weight loss following laparoscopic adjustable gastric banding. Obes Surg. 2013; 23: 1611-1615.
- Werling M, Fandriks L, Vincent RP, Cross GF, le Roux CW, et al. Preoperative assessment of gut hormones does not correlate to weight loss after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2014; 10: 822-828.
- Ness A, Bruce J, Bruce A, Aupperle R, Lepping R, et al. Pre-surgical cortical activation to food pictures is associated with weight loss following bariatric surgery. Surg Obes Relat Dis. 2014; 10: 1188-1195.
- Gerber P , Anderin C, Gustafsson UO, Thorell A. Weight loss before gastric bypass and postoperative weight change: Data from the Scandinavian Obesity Registry (SOReg). Surg Obes Relat Dis. 2016; 12: 556-562.
- Baldridge AS, Pacheco JA, Aufox SA, Kim KY, Silverstein JC, et al. Factors Associated With Long-Term Weight Loss Following Bariatric Surgery Using 2 Methods for Repeated Measures Analysis. Am J Epidemiol. 2015;182: 235-243.
- Manning S, Pucci A, Carter NC, Elkalaawy M, Querci G, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015; 29: 1484-1491.
- Benoit SC, Hunter TD, Francis DM, De La Cruz-Munoz N. Use of Bariatric Outcomes Longitudinal Database (BOLD) to study variability in patient success after bariatric surgery. Obes Surg. 2014; 24: 936-943.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012; 22: 70-89.
- Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16: 1227-1231.
- Snyder B, Nguyen A, Scarbourough T, Yu S, Wilson E. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc 2009; 23: 2302-2306.
- Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB4, Thomas D, et al. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond). 2015; 39: 1109-1113.
MedDocs Publishers
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.