

| Received | : | Oct 19, 2020 |
| Accepted | : | Nov 23, 2020 |
| Published Online | : | Nov 27, 2020 |
| Journal | : | Annals of Pediatrics |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Cardona MA, López-Marín BE, Restrepo M. Development of a Baby Food Pureed with Probiotic Microorganisms for an Infant from 6 to 12 Months. Ann Pediatr. 2020; 3(1): 1039.
Keywords: Baby; Coagulans; Carbohydrate; Food; Probiotic; Shelf life.
Abbreviations: UHT: Ultra High Temperature.
The infant population and more specifically infants between 6 to 12 months are the most vulnerable to suffering from diseases that jeopardize the immune conditions of their developing organism. To counteract this, this research aimed to: develop a functional food baby pureed for an infant aged 6 to 12 months, using a probiotic strain, where the probiotic strain studied was Bacillus coagulans. The methodology involved the preparation of 3 flavors of baby food pureed such as: ahuyama, carrot and figs, which B. coagulans were added as a functional component. A three-month shelf-life study was determined and evaluated, in which a count was made in the CFU of B. coagulans and its permanence in baby food pureed during this time. It was found that the adequate counting technique is vital for the detection of the microorganism and that carbohydrate content favors its permanence. It was concluded that baby food pureed could have a functional effect seen from the count of B. coagulans and from the study of shelf life as follows: ahuyama up to 90 days, carrot 30 days.
The infant population, and specifically the population under 12 months, is one of the most vulnerable in the health aspect, presenting a great tendency to suffer from gastrointestinal diseases such as diarrhea due to rotavirus or antibiotics, and necrotizing enterocolitis [1], also infections and certain allergies [2,3] the above leads to their health and nutritional status being disadvantaged and therefore their growth and cognitive development, which is why the Food industry has been constantly developing products for them.
Currently, there are investigations where the use of probiotics is highlighted as one of the factors that favors the growth of the intestinal microbiota, which results in an adequate performance of the gastrointestinal side, protecting from acute infectious and even chronic non-communicable diseases. Therefore, the development of functional foods with the use of probiotics for this population allows to promote their concentration and their survival in the digestive system; is one of the great trends worldwide [4], since it favors and it stimulates the intestinal microbiota for the proper functioning of the gastrointestinal tract and the immune system [5].
Among the many definitions of probiotics, we highlight this: “live microorganisms that are beneficial for health as a food supplement” [6].
In order for probiotics to provide healthy benefits, it is essential that the following requirements could keep: there must be at least one million viable probiotic organisms per gram of a product, that is to say 107 Colony Forming Units g-1 (CFU), also they must be from human origin, non-pathogenic, resistant to destruction by thermal processing, resistant to destruction by secretions from the gastrointestinal tract, capable of colonizing the gastrointestinal tract, producing antimicrobial substances, modulating immune responses and influencing human metabolic activities [7].
These characteristics are fundamental and all must be fully complied by the microorganism that is used in the development of the product, since a breach in any of these can generate negative effects on the person or not produce any of the expected beneficial effects. Because of this, the international standards have settled down in their regulations to follow the document called Guidelines for the Evaluation of Probiotics in Food of the FAO and WHO [8] before requesting a health claim for a food product. This document explains the step by step of all the requirements that a functional food product with probiotics must comply to be initially accepted and evaluated as a health claim. Among the most rigorous entities is the EFSA [9] which requires several clinical studies to be able to accept a dossier, but standards in countries such as Japan allows a functional probiotic food to be classified at various levels, for example: type 1 classified; it is considered to comply with the nutritional aspect, standardized type 2, that is, it complies with a health benefit; just by having fulfilled the requirement number 1 of the FAO / WHO Guidelines for the Evaluation of Probiotics in Food that deals with the evaluation of the identity and safety of probiotic microorganisms [8] and thus be commercialized. Almost the same happens in America with the FDA, which applies to the entire continent, including Colombia, where Resolution 333 of 2011 is followed, which applies to the nutritional label, including probiotics; regulation allows a functional probiotic product to be sold by categorizing it only as a nutraceutical.
Among the microorganisms capable of being used in the food industry and that if it exerts the functional effects described above is Bacillus coagulans. This microorganism was isolated from green malt in 1932 by scientists Horowitz-Wlassowa and Nowotelnow, then japanese scientists named it Lactobacilus sporogenes to later classify it as B. coagulans [10]. Studies have demonstrated and corroborated the probiotic effect of B. coagulans on health, for example, it has been used as an antibiotic [11] in the treatment of hyperlipidemia, adenovirus, influenza, aphthous stomatitis, dental caries, arthritis and hypercholesterolemia [12] in children’s health in the treatment of diarrhea [13] and in the reduction of gastric colic in combination with fructooligosaccharides [14] The ability to form spores in B. coagulans is what allows it to survive high temperatures and to withstand the biochemical conditions present in the human gastric environment without destroying the probiotic benefits [15]. This work aimed to develop a baby food pureed with a functional effect at the gastrointestinal level for the lactating population between 6 and 12 months, with the use of B. coagulans; using as matrices 1 fruit (breva, Ficus carica) and 2 vegetables (ahuyama, Cucurbit maximum and carrot, Daucus carota).
B. coagulans used was that produced by the Sabinsa Corporation, a product patented as a GRAS product [16].
The fruits, vegetables and all the materials for making the baby food pureed were purchased from a chain supermarket.
The method used for product sanitization was UHT pasteurization, which was done in a tubular pasteurizer reference HT-220, Omve Brand [17,18]. The process was replicated across all flavor batches.
Reception of the vegetable or fruit, selection and classification of the same, washing, disinfection and peeling, chopped, cooked (fruit + water + sugar) at 90ºC for 35 minutes, liquefied, mixed and adding pectin, adding B. coagulans previously diluted according to the manufacturer’s instructions and finally sanitizing the baby food pureed by using UHT.
The main physicochemical parameter was the pH, to assess the stability of the baby food pureed and measure whether changes in it could interfere with the viability of the probiotic. This was done with an Ohaus brand pH meter, which was always precalibrated with pH 7 and pH 4 solutions.
Microbiological analysis, for pathogens and for B. coagulans
In order to determine the asepsis of the baby food pureed processed by UHT, the safety was evaluated through microbiological analysis of the pathogenic microorganisms required by the regulations and resolution 11488 of 1984 issued by the Colombian Ministry of Health and according to the Colombian Technical Standard NTC 1474, which request the total absence of the following: mesophilic microorganisms, molds and yeasts, Clostridium sulfite reducer, total and fecal coliforms, Bacillus cereus, Salmonella sp, Pseudomona sp, Staphylococcus sp, according to NTC 4519, 4132 method and ISO 7954 standard, 4834, 4458, 4679, 4574, 5594, 4779.
A study of the viability of B. coagulans was carried out, as it is the main critical parameter over time to determine the functionality of the baby food pureed [15]. The direct method of Shelf life was worked taking into account, the Guide for the development and presentation of stability studies of conventional medicines of the Ministry of Social Protection update year 2009 and the method of accelerated shelf life (19). In this method, 35 samples for each batch of baby food pureed flavor were conditioned to storage conditions at 40ºC ± 2 and relative humidity 75% ± 5% during the times in days: 0, 15, 30, 45, 60, 75 and 90. Every 15 days starting with time 0, the laboratory analyzes indicated in the planning of activities related to the shelf life study were carried out.
The experimental design for the study of the shelf life of B. coagulans is shown in Table 1.
Two techniques were used to count B. coagulans. The first technique used to carry out the viability study of B. coagulans was carried out under the following conditions: 10 ml of the sample was diluted in 90 ml of peptone water, 1 ml was taken from this solution and it was dispensed into a assay containing 9 ml of peptone water; and so on until 3 dilutions were obtained, which were subcultured as follows: in approximately 20 ml of the gelled agar, 100 μL of the corresponding dilution were added, leading to incubation in a CO2 environment at 37°C between 42 and 72 hours. After this time, the respective readings of the samples were performed according to the experimental design.
Second technique [20] used as reagents: culture medium: yeast Glucose Extract Agar (GYE) composed of: yeast extract 5.0g, peptone (casitone) 5.0g, D-glucose 5.0, potassium acid phosphate (K2HPO4) 0.5g , potassium diacid phosphate (KH2PO4) 0.5g, and magnesium sulfate 0.3; trace mineral solution 1mL, distilled water that made up to one liter; agar (added after adjusting the pH between 6 and 6.3) and the trace mineral solution that consisted of NaCl 500 mg, FeSO4.7H2O 900 mg, MnSO4.5H2O 800 mg, ZnSO4.7H2O 80mg, CuSO4.5H2O 80 mg, CoSO4.7H2O 800 mg and distilled water that reached 50 mL; all of them transferred to a 50 mL volumetric flask dissolving with a small amount of distilled water and then the volume was completed, the solution was refrigerated at a temperature of 4°C.
To obtain the test solution with a 1: 250 dilution, 10 grams of each baby food pureed made with B. coagulans were taken, which was homogenized and 2 mL of sterile saline was added, the pH was adjusted between 6.0 - 6.3 and was taken to an ultrasound bath for 10 to 15 minutes. Subsequently, this mixture was placed in a sterile and dry 250 mL volumetric flask and the volume was completed with sterile saline. With this solution, serial dilutions were made in eppendorf tubes containing 9 mL of sterile saline, until a theoretical CFU number of the dilution between 30 to 300 colonies per plate was obtained, the dilution was transferred to 1 mL in five Petri dishes for subsequent seeding in a water bath at 75ºC for 30 minutes, to then be immediately cooled to 45ºC, then 15 to 20 mL of sterile GYE agar (at 45ºC) were added to each Petri dish, mixing carefully until to obtain a completely solidified medium, the box was inverted and incubated at 37°C for 48-72 hours. Incubation was carefully monitored. After incubation, colony forming units were counted using colony counts. Only plates with a count between 30 and 300 colonies were considered.
Colony numbers for all plates were averaged and multiplied by the dilution factor. This represents the count of viable B. coagulans spores per gram of finished product. The following form of calculation was applied:
Viable spore count Bacillus coagulans / g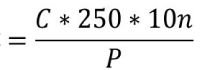
C: # of colonies counted in the Petri dish, 10n: dilution factor where a count was found between 30 and 300 CFU and P: is the weight taken from the sample in grams.
To ensure that B. coagulans was in the product prior to heat treatment, a count analysis of B. coagulans was performed on all three baby food pureed flavors prior to thermal UHT sanitizing process.
Descriptive statistics (averages, standard deviations and coefficients of variance) of the microbiological analyzes for pathogens were performed. Statistical and Excel were used for statistical analysis of the data.
The microbiological results pertinent to each baby food pureed showed the absence of each aforementioned pathogen in CFU / mL units, indicating that it is a suitable food to be supplied to the child population, without health repercussions due to pathogenic microbial contamination after undergoing the sanitization process by UHT.
Result analysis count B. coagulans in the study of shelf life with the first technique.
Results with technique 1, it could be observed that there were only specific days in which B. coagulans content was reported, in the ahuyama on day 0 (1750350 CFU), for the fig on day 45 (2173593 CFU) and the carrot on day 75 (490350 CFU).
The report of the shelf life values of B. coagulans with the first technique indicated a behavior that is not consistent with the growth or death of a microorganism, where initially there is no presence of this, but with time in some of the products growth was seen and then, again there was no presence, the above leads to think about a relationship between the pH and the growth or death of the probiotic. Next, graph 1 shows this relationship between the pH of the baby food pureed and the content of B. coagulans.
In graph 1, very slight pH variations can be observed in the three baby food pureed flavors, which with the Anova test could lead to the conclusion that the pH values of the three compotes are very similar, since the P value obtained was (0.054); indicating that the pH did not have statistically significant variations in time between each product, therefore this parameter is not the cause of a possible decrease of B. coagulans in the product and could not be the cause that affecting the survival of the probiotic.
With technique two (see Table 2), the reported microbiological counts of B. coagulans indicated the presence of the probiotic during the whole study of shelf life.
Among the factors to be analyzed was the count of B. coagulans, which is characterized by being able to withstand practically extreme conditions of temperature, pH, composition of nutrients and storage conditions [21]. This is due to the formation of spores of this probiotic, which allows it to behave in a capsule that gives it the possibility of protecting itself against these extreme conditions, including gastric acids and bile, and thus being able to reach the large intestine intact and exert its action [22]
In the search to determine that it could affect the viability over time of B. coagulans, it is brought up that possibly the low concentration of nutrients provided by the fruits and vegetables with which the baby food pureed were made, may be the reason, since the carbohydrates such as simple sugars are the main nutrients that help their proliferation and maintenance of microorganisms, in addition, the study carried out by Majeed, Côté among others [23] and [15] where they used the microorganism (B. coagulans) in bread, indicated that the carbohydrate content in this product was between 0.11% and 88.81%, likewise a bread with probiotic made and marketed in Colombia whose carbohydrate content between 66 and 100% carbohydrates maintains the viability of the microorganism [16] this compared to the sugar content of the baby food pureeds whose content was 19.9% in ahuyama, 15.8% in fig and 15.43% in carrot, which it was supremely low; the aforementioned indicates that the B. coagulans in these products did not have enough substrate to grow, proliferate and maintain themselves for a longer time, as indicated by Arena 2014, saying in their work that the survival of the microorganism depends largely on the matrix of food [24] since from the technological point of view it is the one that can most affect the functionality of a probiotic [25] In this research, it can be said that, a great advance was made when it was determined that a factor to be improved in all food development with the use of this probiotic; is the use of sugars similar to that found by others such as Srisuvor et al., who have already worked with vegetable matrices, finding out that in the use of three different banana species, each one provided nutritional substances whose effect could be more beneficial for one probiotic than for another, also favoring prebiotic activity and providing the carbohydrates required for its viability [26].
It can be concluded that the higher viability of B. coagulans in the ahuyama flavor baby food pureed compared to the other two flavors, could be due to the nature of the fruit, whose fiber and calorie content is higher in relation to the other two.
Baby food pureed can have a functional effect seen from the count of B. coagulans and from the study of shelf life as follows: ahuyama up to 90 days, carrot 30 days.
It is pertinent when preparing a food product with this probiotic to guarantee an adequate supply of sugars and little or no variability in pH, thus because to improve the shelf life of the microorganism and at the same time to allow the functional effect to be exerted in the child population.
We always work towards offering the best to you. For any queries, please feel free to get in touch with us. Also you may post your valuable feedback after reading our journals, ebooks and after visiting our conferences.