- Case Report
- |
- Open Access
- |
- ISSN: 2637-9627
COVID-19 Associated Acute Disseminated Encephalomyelitis (ADEM) in a Child
- Patrick Fueta*;
- Department of Pediatrics, Driscoll Children’s Hospital, Corpus Christi, Texas, USA.
- Oghogho Igbinoba;
- Department of Pediatrics, Driscoll Children’s Hospital, Corpus Christi, Texas, USA.
- Po-Yang Tsou;
- Department of Pediatrics, Driscoll Children’s Hospital, Corpus Christi, Texas, USA.
- Marcos Valdez;
- Department of Pediatrics, Driscoll Children’s Hospital, Corpus Christi, Texas, USA.
- Sonia Mathew
- Department of Pediatrics, Driscoll Children’s Hospital, Corpus Christi, Texas, USA.

| Received | : | Mar 07, 2022 |
| Accepted | : | Mar 24, 2022 |
| Published Online | : | Mar 28, 2022 |
| Journal | : | Annals of Pediatrics |
| Publisher | : | MedDocs Publishers LLC |
| Online edition | : | http://meddocsonline.org |
Cite this article: Fueta P, Igbinoba O, Tsou PY, Valdez M, Mathew S. COVID‐19‐Associated Acute Disseminated Encephalomyelitis (ADEM) in a Child. Ann Pediatr. 2022; 5(1): 1095.
Abbreviations: COVID-19: Coronavirus Disease 19; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; CNS: Central Nervous System; ADEM: Acute Disseminated Encephalomyelitis; ED: Emergency Department; OSH: Outside Hospital; CBC: Complete Blood Count; CMP: Complete Metabolic Panel; UA: Urinalysis; PCP: Primary Care Provider; PCR: Polymerase Chain Reaction; IV: Intravenous; ESR: Erythrocyte Sedimentation Rate; RBC: Red Blood Cell; MS: Multiple-Sclerosis; MOG: Myelin Oligodendrocyte Glycoprotein (MOG).
Abstract
We report an 8-year-old girl with COVID-19 infection and acute disseminated encephalomyelitis (ADEM). She presented with 2 weeks of fatigue, headache, malaise, and new onset of unsteady gait. She was positive for COVID-19 infection on the respiratory PCR panel with CSF analysis negative for direct infection. Brain MRI demonstrated acute demyelinating lesions on bilateral cerebral hemispheres and right cerebellar peduncle. She received 5-day high-dose intravenous methylprednisolone with significant improvement of neurologic symptoms. This case report highlights the association between ADEM and COVID-19 infection in children. Pediatricians should be aware of the severe spectrum of neurologic complications associated with COVID-19 infection.
Introduction
COVID-19 has led to an ongoing worldwide pandemic and is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1,2]. Confirmed cases of COVID-19 have surpassed 359 million globally [3]. COVID-19 is relatively new, thus, the exact range of illness both in the adult and pediatric population is yet unknown. COVID-19 has a wide scope of symptomatic presentations including headache, fever, cough, anosmia, and loss of taste. Pneumonia leading to respiratory distress is the most common cause of morbidity and mortality of COVID-19 [2,4]. Other established complications of COVID-19 that have been reported in literature include cardiomyopathy, hematologic dysfunction, and liver injury presumably secondary to cytokine storms. A multisystem inflammatory syndrome due to immune dysregulation resulting in cardiac circulatory dysfunction among other presentations is one of the most serious comorbidities associated with COVID-19 disease that has been identified in the pediatric population to date [4].
ADEM is a demyelinating disease of the central nervous system associated with multifocal neurologic symptoms and encephalopathy [5]. The incidence of ADEM is around 0.2-0.5 per 100,000 children [6,7]. It is theorized to be due to a cell-mediated delayed hypersensitivity reaction commonly triggered by an environmental or infectious stimulus in genetically susceptible individuals. Patients may experience a rapid onset of a wide range of neurologic symptoms (e.g., lethargy, confusion, and ataxia) as a result of demyelination of Central Nervous System (CNS) neurons, typically followed by a repair phase resulting in the remyelination of the neurons [5]. Recent literature has associated ADEM with COVID-19 in adult patients [8].
ADEM as a rare complication of COVID-19 infection in pediatric population sparingly reported in literature. Recognizing that ADEM is a possible manifestation of COVID-19 in the pediatric population is critical to ensuring the best possible outcomes. This case report highlights the previously unreported presentation of ADEM in a pediatric patient, the initial diagnostic dilemma, and the subsequent approaches taken to arrive at a diagnosis.
Case presentation
Our patient is a previously healthy 8-year-old female who presented to the Emergency Department (ED) at our facility with complaints of progressive weakness. Mother reported that symptoms began 13 days prior to presentation. On day 3 after the onset of symptoms, the patient complained of associated malaise, headache, and poor appetite. Upon developing these symptoms, she presented to the ED at an Outside Hospital (OSH) and COVID-19 test, Complete Blood Count (CBC), Complete Metabolic Panel (CMP), and Urinalysis (UA) were performed. Mom reported that the patient’s UA was suggestive of a Urinary Tract Infection (UTI), and other tests were unremarkable. Subsequently, she was prescribed a 7-day course of oral cephalexin and discharged from the ED. On day 9, she visited her Primary Care Provider (PCP) due to persistent weakness despite adequate antibiotic compliance. A respiratory Polymerase Chain Reaction (PCR) was ordered, and she was advised to complete the antibiotics course. On day 11, weakness persisted and she developed a new onset of right lower quadrant abdominal pain. She presented to our ED where she was worked up for acute appendicitis however, laboratory workup and abdominal ultrasound was negative for acute appendicitis. After receiving a bolus of Intravenous (IV) fluid, the patient had full resolution of right lower quadrant abdominal pain and she was discharged from the ED. Two days later, the patient’s mother was called by her PCP, she was informed that the respiratory PCR was positive for mycoplasma and the patient was placed on oral azithromycin. The patient’s weakness and fatigue worsened through the course of the day with the patient being unable to stand or ambulate as reported by the mother, and she had 2 episodes of non-bilious, non-bloody emesis. Of note, two weeks prior to the onset of her symptoms, the patient was in contact with her father who reported positive for COVID-19.
Mother reported that the patient was sent to live with her grandmother to prevent her from contracting COVID-19 while they quarantined until resolution of symptoms.
On day 13, the patient revisited the ED due to persistent symptoms, physical examination findings were positive for Kernig’s and Brudszinki’s signs, exaggerated deep tendon reflexes, and wobbly gait. Her cranial nerves II-XII and muscle strength in both upper and lower extremities were within normal limits with intact sensation to light touch, proprioception, and vibration. The patient was admitted for investigation and treatment of the underlying cause of presentation. The laboratory workup showed leukocytosis with a WBC of 15,400 th/uL with predominant neutrophilia (82.7%) and elevated ESR (26 mm/hr). CSF analysis showed slightly elevated glucose of 77 mg/dL, total protein of 67 mg/dL, cell count showed elevated WBC of 91 cells/uL, elevated RBC of 3 cells/uL, elevated neutrophils of 20% and elevated monocytes of 4%. Other tests were negative.
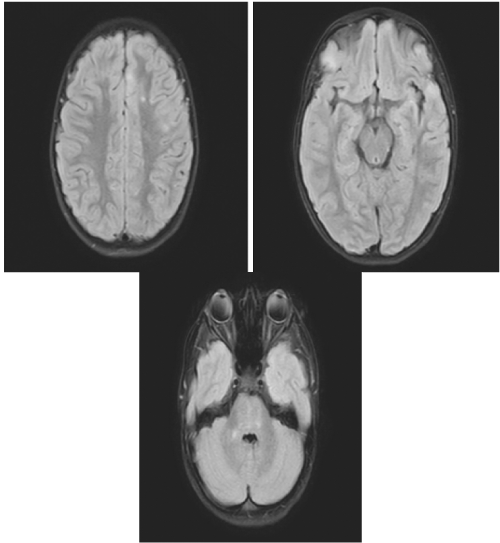
On day 1 of admission (day 14 from symptom onset), her nausea and emesis subsided; however, she continued to complain of weakness. She endorsed back pain from LP, otherwise, she had no additional complaints with unchanged physical examination findings. Due to a high index of suspicion for differential diagnosis of Postinfectious encephalitis, and Acute Disseminated Encephalomyelitis (ADEM), pediatric neurology was consulted and further tests consequently ordered, including respiratory PCR, COVID-19 antibodies, meningitis/encephalitis panel, CSF PCR for COVID-19, and MRI of the brain and spine with and without contrast. Respiratory PCR was positive for COVID-19 with positive COVID-19 IgG. Of note, patient’s CSF PCR for COVID-19 PCR were also sent and returned negative. MRI demonstrated “bilateral punctate areas of increased T2 signal in both cerebral hemispheres and in the right cerebellar peduncle compatible with the characteristic finding of ADEM” (Figure 1).
Figure 1: MRI demonstrating bilateral punctate areas of increased T2 signal in both cerebral hemispheres.
The patient was started on a 5-day course of high dose Intravenous (IV) methylprednisolone at 600 mg daily and physical therapy. Upon completion of IV methylprednisolone therapy, her oral intake, gait/balance, and jitteriness improved, although not at her baseline from an ambulation standpoint. She was subsequently discharged home in stable condition on oral prednisone for 5 days, to follow-up with Neurology and with outpatient physical therapy. At the 4 month follow-up visit with neurology, the MRI of the spine ordered was a normal study, and the patient had complete resolution of weakness and unsteady gait. The patient was subsequently cleared from a neurological standpoint.
Discussion
ADEM secondary to COVID-19 in a child is a rare complication secondary to COVID-19 infection. Our study is important as it demonstrated the neurological complications associated with COVID-19 pandemic with global confirmed case numbers surpassing 90 million. Furthermore, it showed the efficacy of pulse corticosteroids for treating a child with ADEM secondary to COVID-19.
COVID-19 is associated with various neurologic complications, ranging from anosmia, Guillain Barre Syndrome, acute flaccid myelitis, to ADEM. ADEM is a demyelinating disease of the central nervous system associated with various neurologic symptoms [9]. Clinical symptoms of ADEM include encephalopathy (e.g., altered mental status), pyramidal signs, acute hemiparesis, cerebellar ataxia, cranial neuropathy, as well as transverse myelitis [10]. Lumbar puncture with CSF analysis is required to rule out infectious causes and for identification of pleocytosis and oligoclonal bands [10]. Testing for serum autoantibodies (e.g., myelin oligodendrocyte glycoprotein, aquaporin-4 IgG) are also indicated to rule out other demyelinating diseases of the central nervous system. The radiographic characteristics included bilateral deep and subcortical white matter lesions on T2-weighted and FLAIR sequence MRI [10]. ADEM is typically preceded by a viral illness. This suggests ADEM is an autoimmune disorder with autoantibodies attacking host myelin antigens that may have shared antigenic determinants with preceding infectious pathogens. Similar to others’ findings [11-13], our negative result on CSF viral PCR panel and bacterial culture suggested the underlying pathogenesis of ADEM is not directly resulting from viral toxicity or infection. Reported associated pathogens included Coxsackie virus, cytomegalovirus, Epstein-Barr virus, herpes simplex, HIV, and coronavirus. Recently, the newly emerged COVID-19 has been associated with ADEM in adults [14]. Several case reports have described patients with a history of COVID-19 infection who had clinical and neuroimaging findings compatible with ADEM [12,13,15]. Yeh et al. also reported that coronavirus could lead to ADEM [16]. The treatment for ADEM associated with COVID-19 included high-dose steroids, intravenous immunoglobulin, and/or plasma exchange with variable outcomes [17]. While the majority of patients recover without lingering neurologic sequelae, occasionally patients may have persistent neurologic deficits and even mortality. It is reported that those with hemorrhagic changes in the MRI are associated with worse outcomes. In our case, our patient responded well to the steroid pulse treatment. Further studies will be needed to investigate which population might need more treatment in addition to pulse steroids.
The pathophysiology of the neurologic complications associated with COVID-19 were hypothesized not just from the direct toxic effects of the virus, dysfunction of the Renin-Angiotensin System (RAS), but also the profound systemic response to the infection [17]. The high level of circulating cytokines may lead to endothelitis as well as thrombophilia. The above-mentioned effects of dysregulated immune response are particularly important in the pediatric population [17]. MIS-C, a multisystem inflammatory status mediated by cytokine storms with presentation similar to Kawasaki disease. It might be that children are more susceptible to immune-mediated pathologies (e.g., MIS-C, ADEM) secondary to COVID-19 infection. As the COVID-19 pandemic evolves, clinicians should therefore be cautious and aware of the potential immune-mediated complications secondary to COVID-19 infection.
Diseases with presentations similar to ADEM include but are not limited to Multiple Sclerosis (MS) and Myelin Oligodendrocyte Glycoprotein (MOG) antibody-associated diseases. The MRI findings are characteristics for ADEM. Unfortunately, we did not collect enough CSF samples for additional labs to definitively differentiate ADEM from MOG antibody-associated disease. Regardless, the initial treatment for ADEM or MOG antibody-associated disease would be a course of steroid treatment.
Conclusion
This case report illustrates that ADEM could be associated with COVID-19 infection in children. A course of pulse steroids was demonstrated as an effective management for ADEM associated with COVID-19. Given the high infectivity rate of COVID-19, and the tendency for children to experience a dysregulated immune response following COVID-19 infection, pediatricians should be aware of severe neurologic complications such as ADEM to avoid delayed diagnosis and treatment.
Declaration
Data availability: The data used to support the findings of this study are available on reasonable request from the corresponding author
Article summary: This case report discusses the presentation and management of an 8-year-old girl with COVID-19 infection complicated with Acute Disseminated Encephalomyelitis (ADEM).
Author contribution: Dr. Fueta, and Dr. Mathew conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Tsou and Dr. Igbinoba, and Dr. Valdez reviewed the medical record, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): A clinical update. Frontiers of medicine. 2020; 14: 126-135.
- Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020; 92: 568-576.
- Organization WH. Novel Coronavirus ( 2019-nCoV) : Situation report 3. 2020.
- Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020; 55: 2565-2575.
- Esposito S, Di Pietro GM, Madini B, Mastrolia MV, Rigante D. A spectrum of inflammation and demyelination in Acute Disseminated Encephalomyelitis (ADEM) of children. Autoimmun Rev. 2015; 14: 923-929.
- Leake JA, Albani S, Kao AS, Melvin O Senac, Glenn F Billman, et al. Acute disseminated encephalomyelitis in childhood: Epidemiologic, clinical, and laboratory features. Pediatr Infect Dis J. 2004; 23: 756-764.
- Banwell B, Kennedy J, Sadovnick D, DL Arnold, S Magalhaes, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009; 72: 232-239.
- Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, et al. COVID-19-associated acute disseminated encephalomyelitis (ADEM). J Neurol. 2020; 267: 2799-2802.
- Christy A. COVID-19: A Review for the Pediatric Neurologist. J Child Neurol. 2020; 35: 934-939.
- Murthy SN, Faden HS, Cohen ME, Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002; 110: e21.
- Novi G, Rossi T, Pedemonte E, Laura Saitta, Claudia Rolla, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020; 7: e797.
- Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 2020; 13: 597.
- Stonehouse M, Gupte G, Wassmer E, Whitehouse WP. Acute disseminated encephalomyelitis: recognition in the hands of general paediatricians. Arch Dis Child. 2003; 88: 122-124.
- Stuve O, Zamvil SS. Pathogenesis, diagnosis, and treatment of acute disseminated encephalomyelitis. Curr Opin Neurol. 1999; 12: 395-401.
- Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020; 140: 1-6.
- Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004; 113: e73-e76.
- Elkind MS, Cucchiara BL, Koralnik IJ, Rabinstein AA, Kasner SE. Coronavirus Disease 2019 (COVID-19): Neurologic Complications and Management of Neurologic Conditions. 2020.